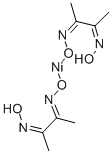
Nickel(II) Dimethylglyoximate [13478-93-8] bis(dimethylglyoximato) nickel(II), Ni(C4H7N2 O2)2, Mr 288.94, is obtained quantitatively as a bright-red precipitate when an alcoholic solution of dimethylglyoxime is added to a neutral or slightly alkaline solution of a nickel(II) salt. This reaction is used in the gravimetric estimation of nickel and is also useful as a spot test for the presence of nickel ions. The structure of this diamagnetic solid shows the nickel(II) ion to be in a squareplanar environment; the dotted lines represent strong intramolecular hydrogen bonds.