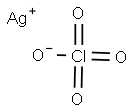Silver perchlorate (chemical formula: AgClO4) is a silver salt of perchloric acid as both a useful source of the Ag+ ion and a catalyst in the organic chemistry. It should be noted that due to the high instability and explosiveness of the anion perchlorate contained in it, it is limited for usage in industry. It is a kind of strong oxidizer and can be used to promoting the oxidation reaction in the organic synthesis. It can also be used as a catalyst for which it can be used to replace a halide with perchlorates to form intermediate in synthesis. It can be manufactured through the heating reaction of the mixture of perchloric acid with silver nitrate, the reaction between barium perchlorate and silver sulfate, or the reaction of perchloric acid with silver oxide.
https://en.wikipedia.org/wiki/Silver_perchlorate
http://www.softschools.com/formulas/chemistry/silver_perchlorate_formula/393/
The silver perchlorate salt, is deliquescent and forms a light-sensitive monohydrate that can be dehydrated at 43 °C and is soluble in a variety of organic solvents. Explosions of silver perchlorate have been reported.
Colorless crystals. Soluble in water; reacts
with explosive violence with many organic sol-
vents; explodes on grinding.
In the explosives industry.
Silver perchlorate is used as a catalyst in organic chemistry. It is an effective reagent for replacing halides ligands with perchlorate. It is also used in the manufacturing of explosives.
reagent type: catalyst
core: silver
Reflux it with *benzene (6mL/g) in a flask fitted with a Dean and Stark trap until all the water is removed azeotropically (ca 4hours). The solution is cooled and diluted with dry pentane (4mL/g of AgClO4). The precipitated AgClO4 is filtered off and dried in a desiccator over P2O5 at 1mm for 24hours [Radell et al. J Am Chem Soc 83 3958 1961]. It has also been recrystallised from perchloric acid. [Caution due to its EXPLOSIVE nature in the presence of organic matter.] Store it in the dark.