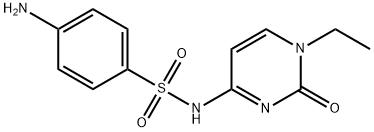
Renoquid,Glenwood,US,1975
The N-(N-acetylsulfanilyl)-1-ethylcytosine used as a starting material is
prepared as follows: To a solution of 333 grams of 3-(ethylamino)propionitrile
in 1,697.3 ml of 2 N hydrochloric acid is added 275 grams of potassium
cyanate, the resulting solution is concentrated under reduced pressure to a
syrup, and the syrup is heated at 90° to 100°C for 6 hours and then
evaporated to dryness at 90° to 100°C under reduced pressure. The residue is
extracted with 1,600 ml of hot absolute ethanol, and the extract is
concentrated to 500 ml and chilled. The crystalline 1-(2-cyanoethyl)-1-
ethylurea obtained is isolated, washed with cold absolute ethanol, and dried,
melting point 88° to 91°C. This intermediate (58.7 grams) is added to a
solution of 11.5 grams of sodium in 500 ml of methanol and the resulting
solution is heated under reflux for 30 minutes. After cooling, the mixture,
containing 1-ethyl-5,6-dihydrocytosine, is treated with a slight excess of
gaseous hydrogen bromide and evaporated to dryness. The residue is
extracted, first with 500 ml, then with 100 ml of hot isopropyl alcohol, the
extracts are combined and chilled, and the crystalline 1-ethyl-5,6-dihydrocytosine hydrobromide obtained is isolated and dried, MP 167.5° to
169.5°C. This salt (88.8 grams) is dissolved in 200 ml of nitrobenzene at
174°C, 22.6 ml of bromine is added over a period of 8 minutes, and the
mixture is kept at 170° to 175°C until hydrogen bromide evolution ceases
(about 15 minutes). Upon cooling, there is obtained crude 1-ethylcytosine
hydrobromide, which is isolated, washed with ether, and dried, MP 170° to
187°C.
This salt is heated at 90° to 100°C with 70 ml of N,N-dimethylformamide and
60 ml of piperidine, and the resulting solution is chilled to give 1-
ethylcytosine, MP 238° to 243°C. A mixture of 10.5 grams of 1-ethylcytosine,
18.6 grams of N-acetylsulfanilyl chloride, and 50 ml of pyridine is stirred at
room temperature for 2 days. The precipitated solid is removed by filtration,
and the filtrate is evaporated at 60°C under reduced pressure to a syrup. The
syrup is triturated with 0.25N hydrochloric acid, and the solid N-(N-acetylsulfanilyl)-
1-ethylcytosine obtained is isolated and dried. This solid is suitable
for use without further purification.
A solution of 65 grams of N-(N-acetylsulfanilyl)-1-ethylcytosine in 380 ml of 2
N aqueous sodium hydroxide is heated under reflux for 1 hour. Upon cooling,
the solution is treated with charcoal, purified by filtration, and acidified with
acetic acid. The solid N-sulfanilyl-1-ethylcytosine that precipitates is isolated,
washed with water, and dried, MP 166.5° to 168°C following successive
crystallizations from butyl alcohol and from methanol.