Irriten,Tosse,W. Germany,1981
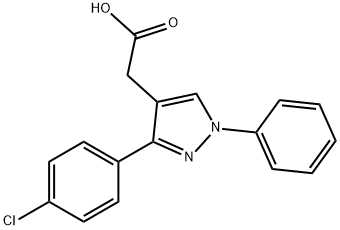
17.6 g 1-phenyl-3-(p-chlorophenyl)-pyrazol-4-acetonitrile and 180 ml 25%
aqueous hydrochloric acid were mixed and heated to the boiling temperature
under reflux for 6 hours. To the mixture was then added dropwise
concentrated aqueous sodium hydroxide until the pH of the mixture reached a
value in the range from 3 to 5. The free pyrazol-4-acetic acid precipitated
thereby was filtered off, redissolved in dilute aqueous sodium hydroxide, the
solution cleared by treatment with activated carbon, and the pyrazol-4-acetic
acid precipitated by acidifying the solution by the addition of dilute mineral
acid, sulfuric acid. The filtered acid was crystallized from a mixture of ethanol
and water. 17.1 g 1-phenyl-3-(p-chlorophenyl)pyrazol-4-acetic acid, melting at
148°C to 150°C, were obtained, representing a yield of 91%.