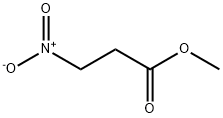
Methyl 3-Nitropropanoate is used to nitroaldol additions, condensation reactions, and Michael additions involving the NO2-substituted
carbon; the α-carbonyl carbon in dilithionitronate enolates of the esters undergoes alkylations, double
alkylations, and aldol and Michael additions; elimination of HNO2 from the products gives α,β-
unsaturated esters with substituents in either the β- or the α-position, thus making the reagent a
synthetic equivalent of acrylate anions with d3 or d2 reactivity; precursor to nitrile oxide for [3 + 2]
cycloadditions; 3-nitropropanoyl chloride can be used for enolate acylation and five-membered ring
annulation.