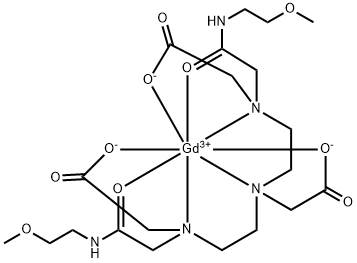
The contrast agent gadoversetamide was launched in the US as a solution for i.v.
injection to be used prior to magnetic resonance imaging (MRI) in patients with anomalous
blood-brain barrier or anomalous vascularity in the central nervous system or in the liver.
This chelate obtained by complexation of paramagnetic gadolinium(III) ion and DTPA-bis-
(methoxyethylamide) ligand enhances visualization of lesions including tumors in the brain,
spine and liver due to the effect of Gd(III)-water interaction on the proton magnetic
relaxation of water. The small molecular size gadoversetamide belongs to the class of
extracellular contrast agents. A phase III, multicenter, double-blind, parallel group trial
showed that gadoversetamide was well tolerated and as efficient as gadopentetate
dimeglumine (Schering) in hepatic magnetic resonance imaging of patients with suspected
liver pathology.
Gadoversetamide is a gadolinium containing complex used as a contrasting imaging agent.
Diagnostic aid (paramagnetic, brain disorders; spine disorders.
ChEBI: A gadolinium coordination entity that consists of Gd3+ coordinated to 3,6,9-triazaundecadiamide in which each of the amide nitrogens is substituted by a 2-methoxyethyl group and in which the nitrogens at positions 3, 6, and 9 are each
ubstituted by carboxylatomethyl group. The gadolinium is coordinated to the three tertiary amino groups as well as to the carboxylate groups. A white odourless powder that is freely soluble in water, gadoversetamide has paramagnetic properties and is used
s a contrast agent in magnetic resonance imaging. It distributes mainly in extracellular fluid, but does not cross the blood-brain barrier. It is used particularly in imaging the brain, spine and liver.
A stirred suspension of diethylenetriaminepentaacetic acid dianhydride (10.8
g, 0.030 mole) in 100 ml of isopropanol was treated with 2-
methoxyethylamine (5.0 g, 0.067 mole). The entire mixture was heated at
50°C for 4 hours in a water bath. The pale yellow solution was filtered
through a medium porosity sintered glass funnel to remove undissolved
impurities, and the filtrate was taken to dryness under reduced pressure. The
resulting amorphous foam was dried (vacuum desiccator) at ambient
temperature for 18 hours. The yield of the N,N"-bis[N-(2-methoxyethyl)-
carbamoylmethyl]diethylenetriamine-N,N',N"-triacetic acid 14.4 g (93.5%).
A mixture of gadolinium (III) oxide (3.3 g, 0.0091 mole) and N,N"-bis[N-(2-
methoxyethyl)-carbamoylmethyl]diethylenetriamine-N,N',N"-triacetic acid
(10.2 g, 0.020 mole) in H2O (100 ml) was heated at 60-65°C for 3 hours in a
water bath. The pale yellow homogeneous solution was filtered through a fine
porosity sintered glass funnel to remove undissolved impurities and the clear
filtrate was poured into acetone (2 L). The heterogeneous mixture was stirred
for 5 min and allowed to stand at ambient temperature for 30 min. Aqueous
acetone was decanted off and the resulting gummy residue was dissolved with
methanol (150 ml). The solution was concentrated under reduced pressure
and the complex was precipitated from the solution by adding it to more
acetone (1 L). The amorphous precipitate was collected, washed with acetone
and dried. The yield of {N,N"-bis[N-(2-methoxyethyl)-carbamoylmethyl]
diethylenetriamine-N,N',N"-triaceto}gadolinium (III) was 11.2 g (80.7%). The
pale cream solid was crystallized from a mixture of methanol and
tetrahydrofuran to give a colorless solid. It was 97.4% pure by HPLC. For {N,N"-bis[N-(2-methoxyethyl)-carbamoylmethyl]diethylenetriamine-N,N',N"-
triaceto}gadolinium (III) calculated: Gd, 22.88%, found: Gd, 22.52%.