hexagonal prisms; decomposes at white heat; refractive index 1.564 (20°C) [HAW93] [MER06]
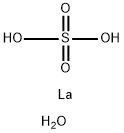
White hexagonal crystals; density 2.82 g/cm3; slightly soluble in cold water, solubility decreasing with temperature; insoluble in ethanol; dehydrates in the air at 400°C.
Lanthanum sulfate is used to prepare many lanthanum compounds.
Lanthanum sulfate is prepared by dissolving lanthanum oxide, hydroxide or carbonate in sulfuric acid, followed by crystallization.
La2O3 + 3H2SO4 → La2(SO4)3 + H2O
La2(CO3)3 + 3H2SO4 → La2(SO4)3 + 3CO2 + 3H2O
.