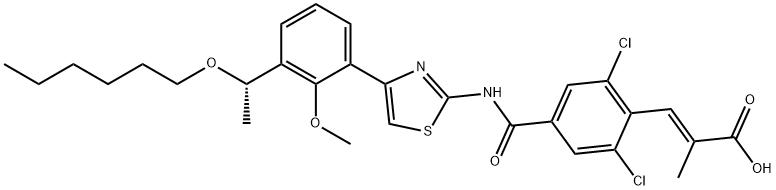
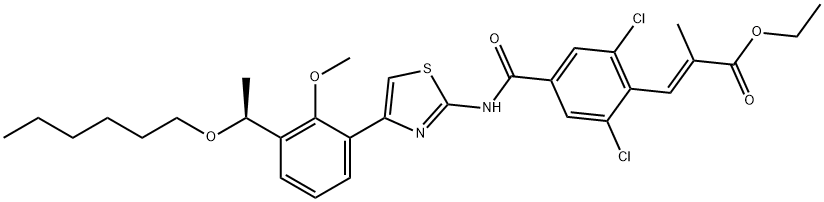
GENERAL STEPS: In a three-necked flask equipped with a mechanical stirrer, 100 mL of tetrahydrofuran (THF) and 25.2 g (40.7 mmol) of ethyl (S,E)-3-(2,6-dichloro-4-((4-(3-(1-(hexyloxy)ethyl)-2-methoxyphenyl)thiazol-2-yl)carbamoyl)phenyl)-2-methacrylate were added. Subsequently, 1.64 g of sodium hydroxide and 100 mL of water were added and the reaction mixture was heated to 60 °C with continuous stirring for 5 hours. The progress of the reaction was monitored by thin layer chromatography (TLC) and after confirming complete consumption of the raw materials, the reaction mixture was cooled to room temperature. Most of the organic solvent was evaporated under reduced pressure and then the pH was adjusted to 2-3 by slow dropwise addition of 2 N dilute hydrochloric acid, at which point a yellow solid precipitated. The solid product was collected by filtration and washed 2-3 times with methyl tert-butyl ether to give finally (S,E)-3-(2,6-dichloro-4-((4-(3-(1-(hexyloxy)ethyl)-2-methoxyphenyl)thiazol-2-yl)carbamoyl)phenyl)-2-methylacrylic acid in white solid powder form in a yield of 23.8 g and 99% yield.