A solution of N-benzylmethylamine 7.27 g (0.06 mole) in 25 ml of acetonitrile
is added dropwise during 10 min to a solution of 8.76 g (0.03 mole) of 3-(2-
bromoacetyl)methanesulfonanilide (M.P. 118-121°C) in 100 ml of acetonitrile.
External cooling is employed to maintain a reaction temperature of 10°C
during the addition. The cooling bath is then removed and the solution stirred
for an additional 20 min. Concentration of the reaction mixture yields a yellow
oil which is dissolved in 300 ml of ether and washed with water to remove
byproduct N-benzylmethylamine hydrobromide. The ethereal solution is dried
over magnesium sulfate and the solvent distilled leaving a viscous oil. The oil
is purified by dissolving in ether (250 ml), and filtering the ether solution
through diatomaceous earth to remove insoluble colored impurities. The
treated ether solution is diluted with 100 ml of acetonitrile. Treatment of the
ether-acetonitrile solution with an ether solution of methanesulfonic acid yields
3-(2-benzylmethylaminoacetyl)methanesulfonanilide methanesulfonate as a
white precipitate which is collected and washed with 100 ml of 1:1
acetonitrile-ether and dried. It weighs 9.0 g (70%), and exhibits M.P. 197.5-
201°C. It is recrystallized from 96% ethanol, yielding 8.0 g (62.3%) of the 3-
(2-benzylmethylaminoacetyl)methanesulfonanilide methanesulfonate as a
crystalline product, M.P. 206-209°C.
A solution of 31.8 g (0.74 mole) of 3-(2-benzylmethylaminoacetyl)
methanesulfonanilide methanesulfonate in 700 ml of absolute ethanol is
reduced in an atmospheric hydrogenation unit (2 to 5 p.s.i. g positive
pressure) during 24 hours with a 10% palladium catalyst prepared from 320
mg of palladium chloride and 2.0 g of pulverized charcoal. After absorption of
the calculated amount of hydrogen, the catalyst is filtered, the filtrate
concentrated to about 100 ml, mixed with about 500 ml of ether, resulting in
precipitation of a white solid weighing 24.3 g (96%), M.P. 201-203.5°C. Two
recrystallizations from ethanol (35 ml/g of solid) yield the analytically pure 3-
(2-methylamino-1-hydroxyethyl)methanesulfonanilide methanesulfonate, 19.6
g (75%), M.P. 207-209°C.
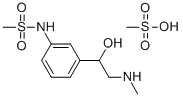
3-(2-Methylamino-1-hydroxyethyl)methanesulfonanilide methanesulfonate,
17.0 g (0.05 mole) is dissolved in 50 ml of 1 N sodium hydroxide, yielding a
yellow solution having pH 8. The water is removed from the solution by
evaporation in vacuo and the residue is treated with several portions of
ethanol which are evaporated to remove last traces of moisture. The bulk of
the sodium methanesulfonate byproduct is then removed from the residue by
dissolving the product in 350 ml of hot absolute ethanol and clarifying by
filtration through a diatomaceous earth filter aid. The ethanolic filtrate is
evaporated to dryness. Last traces of sodium methanesulfonate are then
removed by washing the residue with 15 ml of cold water and then with two
15 ml portions of 1:1 cold water-isopropanol, and finally with ether, yielding
7.7 g of 3-(2-methylamino-1-hydroxyethyl)methanesulfonanilide as a white
solid, M.P. 159-161°C.
In practice it is usually used as monomethanesulfonate salt.