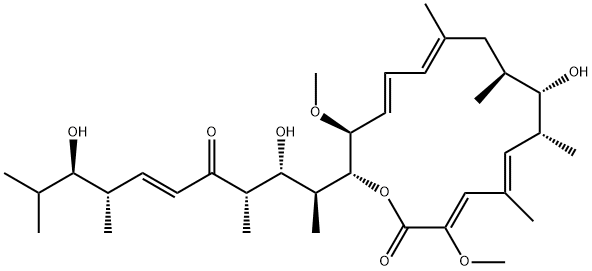
The bafilomycins are fungal plecomacrolide antibiotics with a 16-membered lactone ring. They inhibit the growth of Gram-positive bacteria and fungi. Bafilomycin D is a potent inhibitor of vacuolar H+ ATPases (V-ATPases) that inhibits the V-ATPase from the fungus N. crassa with an IC50 value of approximately 2 nM. It is about 1,000-fold less effective against the K+-dependent ATPase of E. coli.
Bafilomycin D is a member of a potent family of macrocyclic lactones. Bafilomycin D shares the same mode of action as bafilomycin A1 which has been the analogue of choice in cell biology studies of the role of ATPase. Bafilomycin D contains the ring-opened side chain and is a much more stable analogue of bafilomycin A1. Limited availability has restricted a more in depth investigation of this metabolite.
bafilomycin d is a potent inhibitor of vacuolar h+ atpases (v-atpases) with ic50 value of approximately 2 nm for the v-atpase from the fungus n. crassa [1].ion pumps use the energy provided by the hydrolysis of atp to energize ion-transport processes across cell membranes. atpases can be distinguished to p-type, f-type and v-type atpases. p-type atpases have a phosphorylated transitional stage, f-type atpases are primarily used in atp synthesis, and v-type atpases are genetically and functionally related to f-atpases but function only in atp breakdown [1].the bafilomycins are fungal plecomacrolide class macrolide antibiotics isolated from the culture medium of streptomyces sp. and are also high-affinity inhibitors of v-atpases and can be used to study specifically the function of this type of atpase. they inhibited the growth of gram-positive bacteria and fungi. bafilomycin c1 inhibited the enzymatic activity of the na+, k+-atpase with ki value of 11 μmol/l and showed anthelmintic activity against caenorhabditis elegans [1][2].
[1]. drse s, altendorf k. bafilomycins and concanamycins as inhibitors of v-atpases and p-atpases. j exp biol. 1997 jan;200(pt 1):1-8.
[2]. bowman ej, siebers a, altendorf k. bafilomycins: a class of inhibitors of membrane atpases from microorganisms, animal cells, and plant cells. proc natl acad sci u s a. 1988 nov;85(21):7972-6.