Usage And Synthesis
The producing of starting materials 1 and 2:
1). The solution of 16.0 g of bromine in 10 ml of petroleum ether is slowly added to the solution of 6.7 g of allyl cyanide in 30 ml of the same solvent, while stirring and keeping the temperature at about -15°C. After removal of the solvent the oily 3,4-dibromo-butyronitrile is obtained [J.A. C.S. 67, 400 (1945)].
227.0 g of 3,4-dibromobutyronitrile are added dropwise in 5 equal parts to the stirred mixture of 85.0 g of catechol and 50. g of anhydrous potassium carbonate in 100 ml of refluxing acetone each. Another 50.0 g of potassium carbonate are added, followed by a slow addition of another part of nitrile. After 3 more cycles, using 40.0 g of potassium carbonate, 1 part nitrile each and sufficient acetone to allow stirring, the mixture is refluxed for 20 h. It is filtered, the filtrate evaporated, the residue distilled and the fraction boiling at 105°C/0.15 mm Hg collected, to yield the 1,4-benzodioxan-2-yl-acetonitrile [Belgium Pat. No. 643,853-Aug. 14, 1964].
The mixture of 111.0 g of 1,4-benzodioxan-2-yl-acetonitrile, 63.5 ml of sulfuric acid, 160 ml of acetic acid and 160 ml of water is refluxed for 48 h. It is poured on ice, the resulting solid collected to yield the 1,4-benzodioxan-2- yl-acetic acid, melting point 100°C, (recrystallized from benzene-petroleum ether) [Belgium Pat. No. 613,211-July 30, 1962].
The solution of 5.8 g of 1,4-benzodioxan-2-yl-acetic acid in 100 ml of benzene is added dropwise to 16.5 ml of a refluxing, 70% benzene solution of sodium bis(2-methoxyethoxy)aluminum hydride under nitrogen. When addition is complete, the mixture is refluxed for 4 h, cooled and poured slowly into 20 ml of 25% sulfuric acid. After filtration and removal of the solvent, the residue is taken up in methylene chloride, the solution washed several times with saturated aqueous sodium bicarbonate, dried and evaporated, to yield the oily 2-(2-hydroxyethyl)-1,4-benzodioxan.
The mixture of 3.6 g of 2-(2-hydroxyethyl)-1,4-benzodioxan, 5.7 g of ptoluenesulfonyl chloride and 20 ml of dry pyridine is stirred and cooled in an ice bath for 2 h. Ice is then added to the mixture, the resulting solid is filtered off to yield the 2-(2-tosyloxyethyl)-1,4-benzodioxan, melting point 82°-83°C (recrystallized from ethyl acetate-petroleum ether).
2). To the solution of 1.6 g of 4-aminopyridine in 7 ml of dimethylformamide 2.0 g of 2-chloroethylisocyanate are added while stirring and keeping the temperature below 40°C. After 2 h 28 ml of water are added and stirring is continued for 2 h at room temperature. The precipitate formed is filtered off, washed with water, dried to yield the 1-(4-pyridyl)-3-(2-chloroethyl)urea, melting point 120°-122°C, (recrystallized from aqueous ethanol). To the suspension of 2.66 g of 1-(4-pyridyl)-3-(2-chloroethyl)urea in 4 ml of boiling methanol, 2.68 g of 30.8% methanolic sodium methanolate are added while stirring and the mixture is refluxed for 1 h. It is filtered hot, washed with hot methanol, the filtrate evaporated, to yield the 1-(4-pyridyl)-2- imidazolidinone, melting point 204°-207°C, (recrystallized from 90% aqeuous ethanol).
The solution of 5.0 g of 1-(4-pyridyl)-2-imidazolidinone in 45 ml of water is hydrogenated over 0.8 g of 10% ruthenium on carbon at 120°C and 120 atm until the hydrogen absorption ceases. It is filtered, the filtrate evaporated, the residue taken up in chloroform, the solution dried, evaporated to yield the 1- (4-piperidyl)-2-imidazolidinone, melting point 155°-157°C (recrystallized from methylene chloride-petroleum ether).
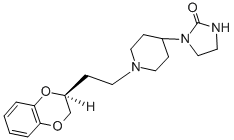
The producing of (cis)-(S)-1-[1-[2-(1,4-benzodioxan-2-yl)ethyl]-4-piperidyl]- 2-imidazolidinone fumarate:
The mixture of 4.9 g of 2-(2-tosyloxyethyl)-1,4-benzodioxan, 2.54 g of 1-(4- piperidyl)-2-imidazolidinone, 5.0 g of anhydrous sodium carbonate and 100 ml of 4-methyl-2-pentanone is stirred and refluxed for 3 days. It is filtered, evaporated, to yield the 1-[1-[2-(1,4-benzodioxan-2-yl)-ethyl]-4-piperidyl]-2- imidazolidinone, melting point 125°C, (recrystallized from isopropanol).
To the solution of 2.0 g of 1-[1-[2-(1,4-benzodioxan-2-yl)-ethyl]-4-piperidyl]- 2-imidazolidinone in the mini-mum amount of ethanol, the saturated solution of 0.78 g of furmaric acid in boiling ethanol is added. The mixture is cooled to 0°C and the precipitate collected, to yield the 1-[1-[2-(1,4-benzodioxan-2-yl)- ethyl]-4-piperidyl]-2-imidazolidinone fumarate, melting point 190°C.
The (cis)-(S)-form may be produced by chromatographic division of isomers of 1-[1-[2-(1,4-benzodioxan-2-yl)ethyl]-4-piperidyl]-2-imidazolidinone fumarate.
1). The solution of 16.0 g of bromine in 10 ml of petroleum ether is slowly added to the solution of 6.7 g of allyl cyanide in 30 ml of the same solvent, while stirring and keeping the temperature at about -15°C. After removal of the solvent the oily 3,4-dibromo-butyronitrile is obtained [J.A. C.S. 67, 400 (1945)].
227.0 g of 3,4-dibromobutyronitrile are added dropwise in 5 equal parts to the stirred mixture of 85.0 g of catechol and 50. g of anhydrous potassium carbonate in 100 ml of refluxing acetone each. Another 50.0 g of potassium carbonate are added, followed by a slow addition of another part of nitrile. After 3 more cycles, using 40.0 g of potassium carbonate, 1 part nitrile each and sufficient acetone to allow stirring, the mixture is refluxed for 20 h. It is filtered, the filtrate evaporated, the residue distilled and the fraction boiling at 105°C/0.15 mm Hg collected, to yield the 1,4-benzodioxan-2-yl-acetonitrile [Belgium Pat. No. 643,853-Aug. 14, 1964].
The mixture of 111.0 g of 1,4-benzodioxan-2-yl-acetonitrile, 63.5 ml of sulfuric acid, 160 ml of acetic acid and 160 ml of water is refluxed for 48 h. It is poured on ice, the resulting solid collected to yield the 1,4-benzodioxan-2- yl-acetic acid, melting point 100°C, (recrystallized from benzene-petroleum ether) [Belgium Pat. No. 613,211-July 30, 1962].
The solution of 5.8 g of 1,4-benzodioxan-2-yl-acetic acid in 100 ml of benzene is added dropwise to 16.5 ml of a refluxing, 70% benzene solution of sodium bis(2-methoxyethoxy)aluminum hydride under nitrogen. When addition is complete, the mixture is refluxed for 4 h, cooled and poured slowly into 20 ml of 25% sulfuric acid. After filtration and removal of the solvent, the residue is taken up in methylene chloride, the solution washed several times with saturated aqueous sodium bicarbonate, dried and evaporated, to yield the oily 2-(2-hydroxyethyl)-1,4-benzodioxan.
The mixture of 3.6 g of 2-(2-hydroxyethyl)-1,4-benzodioxan, 5.7 g of ptoluenesulfonyl chloride and 20 ml of dry pyridine is stirred and cooled in an ice bath for 2 h. Ice is then added to the mixture, the resulting solid is filtered off to yield the 2-(2-tosyloxyethyl)-1,4-benzodioxan, melting point 82°-83°C (recrystallized from ethyl acetate-petroleum ether).
2). To the solution of 1.6 g of 4-aminopyridine in 7 ml of dimethylformamide 2.0 g of 2-chloroethylisocyanate are added while stirring and keeping the temperature below 40°C. After 2 h 28 ml of water are added and stirring is continued for 2 h at room temperature. The precipitate formed is filtered off, washed with water, dried to yield the 1-(4-pyridyl)-3-(2-chloroethyl)urea, melting point 120°-122°C, (recrystallized from aqueous ethanol). To the suspension of 2.66 g of 1-(4-pyridyl)-3-(2-chloroethyl)urea in 4 ml of boiling methanol, 2.68 g of 30.8% methanolic sodium methanolate are added while stirring and the mixture is refluxed for 1 h. It is filtered hot, washed with hot methanol, the filtrate evaporated, to yield the 1-(4-pyridyl)-2- imidazolidinone, melting point 204°-207°C, (recrystallized from 90% aqeuous ethanol).
The solution of 5.0 g of 1-(4-pyridyl)-2-imidazolidinone in 45 ml of water is hydrogenated over 0.8 g of 10% ruthenium on carbon at 120°C and 120 atm until the hydrogen absorption ceases. It is filtered, the filtrate evaporated, the residue taken up in chloroform, the solution dried, evaporated to yield the 1- (4-piperidyl)-2-imidazolidinone, melting point 155°-157°C (recrystallized from methylene chloride-petroleum ether).
The producing of (cis)-(S)-1-[1-[2-(1,4-benzodioxan-2-yl)ethyl]-4-piperidyl]- 2-imidazolidinone fumarate:
The mixture of 4.9 g of 2-(2-tosyloxyethyl)-1,4-benzodioxan, 2.54 g of 1-(4- piperidyl)-2-imidazolidinone, 5.0 g of anhydrous sodium carbonate and 100 ml of 4-methyl-2-pentanone is stirred and refluxed for 3 days. It is filtered, evaporated, to yield the 1-[1-[2-(1,4-benzodioxan-2-yl)-ethyl]-4-piperidyl]-2- imidazolidinone, melting point 125°C, (recrystallized from isopropanol).
To the solution of 2.0 g of 1-[1-[2-(1,4-benzodioxan-2-yl)-ethyl]-4-piperidyl]- 2-imidazolidinone in the mini-mum amount of ethanol, the saturated solution of 0.78 g of furmaric acid in boiling ethanol is added. The mixture is cooled to 0°C and the precipitate collected, to yield the 1-[1-[2-(1,4-benzodioxan-2-yl)- ethyl]-4-piperidyl]-2-imidazolidinone fumarate, melting point 190°C.
The (cis)-(S)-form may be produced by chromatographic division of isomers of 1-[1-[2-(1,4-benzodioxan-2-yl)ethyl]-4-piperidyl]-2-imidazolidinone fumarate.
Preparation Products And Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine