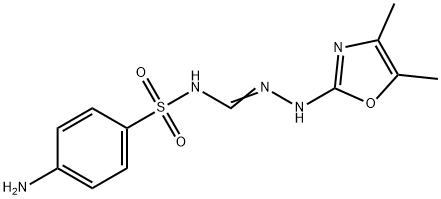
Enterocura,Nordmark,W. Germany,1973
23.9 grams (0.1 mol) of N1-[p-amino benzene sulfonyl]-N3-cyanoguanidine
and 13.2 grams (0.15 mol) of acetoin are thoroughly stirred in a mixture of
120 cc of water and 120 cc of methanol. 25 cc of concentrated hydrochloric
acid are added dropwise with stirring to this suspension at 40°C. A clear
solution is obtained after 30 minutes which solution is kept at 40°C for
another hour. Thereafter, the methanol is distilled off in a vacuum, the
remaining solution is treated with charcoal and the pH of the filtered solution
is quickly brought to 11 by addition of 10% soda lye with quick stirring.
The compound at first precipitated is redissolved at a pH of 11. The solution is
treated another time with charcoal and is filtered. Thereafter, a mixture of
anhydrous acetic acid and water in a proportion of 1:1 is added with stirring
and cooling until a pH of 7 is reached. Thus, the reaction product separates
with crystallization.
For purification, the product is recrystallized from 15 times the amount of a
9:1 mixture of acetone and water. The resulting N1-[p-amino benzene
sulfonyl] -N3-(4,5-dimethyl-oxazolyl-(2)]-guanidine is obtained as colorless
crystals having a MP of 233° to 236°C.