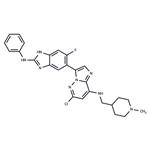
IRE1α kinase-IN-1 is a highly selective IRE1α (ERN1) inhibitor, with an IC50 of 77 nM. IRE1α kinase-IN-1 displays 100-fold selectivity for IRE1α over the IRE1β isoform. IRE1α kinase-IN-1 inhibits ER stress-induced IRE1α oligomerization and autophosphorylation, and also inhibits IRE1α RNase activity (IC50=80 nM)[1].
IRE1α kinase-IN-1 (compound 31) prevents endoplasmic reticulum stress-induced IRE1α oligomerization and phosphorylation, and inhibits endoribonuclease activity in human cells[1].IRE1α kinase-IN-1 and is very high selectivity with >70% inhibition of only 4/455 kinases. IRE1α kinase-IN-1 inhibits recombinant G547 IRE1α KEN domain pS274 autophosphorylation with an IC50 of 160 nM. IRE1α kinase-IN-1 inhibits tunicamycin-induced GFP-IRE1α foci in HEK293 cells with an IC50 of 0.74 μM. IRE1α kinase-IN-1 Inhibits ATP-site LanthaScreen tracer binding to recombinant dephosphorylated G547 IRE1α KEN with an IC50 of 0.27 μM[1].IRE1α kinase-IN-1 inhibits both tunicamycin- and thapsigargin-induced IRE1α-dependent splicing of XBP1 luciferase fusion mRNA in HEK293 cells with IC50s ranging 0.68-1.63 μM[1].IRE1α kinase-IN-1 (0-20 μM) inhibits IRE1α-dependent XBP1s mRNA expression in H929 cells.IRE1α kinase-IN-1 (0-20 μM) dose-dependently inhibits tunicamycin-induced expression of XBP1s in NCI-H929 cells[1].
![Imidazo[1,2-b]pyridazin-8-amine, 6-chloro-3-[6-fluoro-2-(phenylamino)-1H-benzimidazol-5-yl]-N-[(1-methyl-4-piperidinyl)methyl]- Structure](/CAS/20210111/GIF/2328097-41-0.gif)