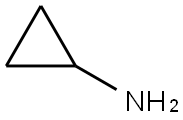
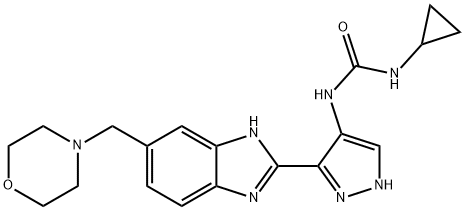
7-Morpholin-4-ylmethyl-2,5,4-dihydro-1,2,4,5a,10-pentaaza-cyclopenta[a]fluoren-5-one (0.797 kg, 2.46 mol, 1.0 eq.) and 1-methyl-2-pyrrolidinone (2.40 L, 3.0 v/v) were added slowly to a flanged flask equipped with a mechanical stirrer, a condenser and a thermometer in a flammable atmosphere protected by nitrogen at 15 to 30 °C. The mixture of cyclopropylamine (0.279 kg, 4.88 mol, 0.35 eq.) and 1-methyl-2-pyrrolidone (2.40 L, 3.0 v/v) were added slowly at 15 to 30 °C under nitrogen. Cyclopropylamine (0.279 kg, 4.88 mol, 0.35 eq.) was slowly added at 15 to 30 °C under the protection of nitrogen. The reaction mixture was heated to 95 to 105 °C and stirred at this temperature for 16 to 24 h. The completion of the reaction was monitored by 1H NMR. Upon completion of the reaction, the mixture was cooled to 10 to 20 °C, ethyl acetate (8.00 L, 10.0 v/v) and saturated NaHCO3 aqueous solution (2.50 L, 3.0 v/v) were added, and the mixture was stirred for 2 to 5 minutes and then partitioned. The organic phase was washed with saturated aqueous NaHCO3 (5.00 L, 1.0 volume) for 25 to 35 minutes, filtered and the filter cake was washed with ethyl acetate (0.40 L, 0.5 volume). The filter cake was retained and the filtrate was transferred to a partitioning funnel for layering and the process was repeated three times. The retained solid was combined with the organic phase and the mixture was concentrated to dryness under vacuum at 35 to 45 °C. The concentrate was dissolved in isopropanol (8.00 L, 10.0 v/v) at 45 to 55 °C, activated charcoal (0.080 kg, 0.1 eq.) was added, and the mixture was stirred at 45 to 55 °C for 30 to 40 min and then hot-filtered, and the filter cake was washed with isopropanol (0.40 L, 0.5 v/v). Activated carbon (0.080 kg, 0.1 equiv) was added to the combined filtrates and the mixture was stirred at 45 to 55 °C for 30 to 40 minutes before hot filtration and the filter cake was washed with isopropanol (0.40 L, 0.5 v/v). The filtrate and washings were concentrated under vacuum at 35 to 45 °C. To the concentrate was added ethyl acetate (8.00 L, 10.0 v/v) and water (2.20 L, 3.0 v/v), the mixture was stirred for 1 to 2 minutes at 25°C and then partitioned, and the organic phase was concentrated under vacuum at 35 to 45°C. Ethyl acetate (4.00 L, 5.0 vol) was added to the residue and the mixture was concentrated under vacuum at 35 to 45 °C. After repeating this operation once, the residue was stirred at 15 to 25°C for 2 to 20 hours, cooled to 0°C and aged at 0 to 5°C for 90 to 120 minutes before being filtered. The filter cake was washed with ethyl acetate (0.80 L, 1.0 v/v), dried for 15 to 30 min, and the solid was dried under vacuum at 35 to 45 °C to afford the target product 1-cyclopropyl-3-[3-(5-morpholin-4-ylmethyl-1H-benzimidazol-2-yl)-1H-pyrazol-4-yl]urea (0.533 kg, 56.8% yield, 93.20% HPLC purity) as a brown solid. ), a brown solid.
![5H-Pyrazolo[4',3':4,5]pyrimido[1,6-a]benzimidazol-5-one, 2,4-dihydro-8-(4-morpholinylmethyl)-](/CAS/20210305/GIF/896466-73-2.gif)
![5H-Pyrazolo[4',3':4,5]pyrimido[1,6-a]benzimidazol-5-one, 2,4-dihydro-9-(4-morpholinylmethyl)-](/CAS/20210305/GIF/896466-74-3.gif)