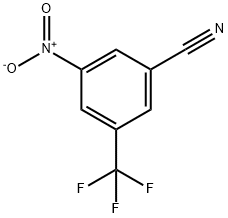
To a solution of dichloromethane (20 mL) containing 3-nitro-5-(trifluoromethyl)benzamide (1.5 g, 6.41 mmol) was sequentially added triethylamine (1.314 mL, 9.61 mmol) and trifluoroacetic anhydride (1.357 mL, 9.61 mmol) at 20 °C. The reaction mixture was stirred continuously at 20°C for 2 hours. The progress of the reaction was monitored by thin layer chromatography (Expanding Agent: Petroleum Ether/Ethyl Acetate=3:1, Rf=0.6) to confirm that the reaction was complete. After completion of the reaction, the mixture was concentrated to give the crude product. The crude product was purified by column chromatography (eluent: petroleum ether/ethyl acetate=3:1, Rf=0.6) to afford 3-nitro-5-(trifluoromethyl)benzonitrile (1.2 g, 5.14 mmol, 80% yield). The structure of the product was confirmed by NMR hydrogen spectroscopy (400 MHz, CDCl3-d): δ 8.71 (s, 2H), 8.24 (s, 1H).