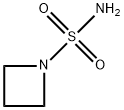
The general procedure for the synthesis of 1-azetidinylsulfonamide using the compound (CAS:1271835-78-9) as starting material was as follows: palladium hydroxide (20%, 350 mg), benzyl (azetidin-1-ylsulfonyl) carbamate (1.49 g, 5.5 mmol), and 1-methyl-1,4-cyclohexadiene (10.7 g, 0.11 mol) of the The mixture was dissolved in methanol (35 mL) and the reaction was stirred at 60 °C under nitrogen protection overnight. After completion of the reaction, the mixture was cooled to room temperature, filtered through a diatomaceous earth pad, and the filtrate was concentrated in vacuum to afford 1-azetidinesulfonamide (437 mg, 58% yield) as a solid product. The product was characterized by 1H NMR (400 MHz, CD3OD) with chemical shifts δ of 2.15 (quintuple peak, 2H) and 3.78 (triplet peak, 4H).The corresponding mass ions were not detected by LCMS analysis.