Usage And Synthesis
To a solution of 11.5 parts lithium aluminum hydride in 100 parts
tetrahydrofurane is added dropwise a solution of 94 parts DL-1-cyano-4-
phenyl-piperidine in 240 parts tetrahydrofurane, at a temperature of about
45°C. After the addition is complete, the reaction mixture is stirred first at the
same temperature for 3 h and 30 min and then refluxed for 1 h. The whole is
decomposed by successive addition of 12 parts water, 9 parts sodium hydroxide 20% and 50 parts water. The mixture is filtered from inorganic
matter. The filter-cake is washed with tetrahydrofurane and the combined
filtrates are evaporated. The oily residue is dissolved in 240 parts 2-propanol
and to this solution are added about 60 parts concentrated hydrochloric acid.
After keeping at room temperature, the precipitated salt is filtered off, washed
with 2-propanol and dried, yielding DL-4-(amino-methyl)-1-[1,4-
benzodioxanyl)methyl]-4-phenylpiperidine dihydrochloride; melting point
272°-278°C as a white amorphous powder.
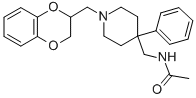
From 4.1 parts DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4- phenylpiperidine dihydrochloride, the free base is liberated in the usual manner and extracted with chloroform. The organic layer is separated, dried and evaporated. The DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4- phenylpiperidine obtained is dissolved in 128 parts anhydrous chloroform. This solution is cooled to 5°C and there is added dropwise a solution of 1.6 parts acetylchloride in 7 parts anhydrous chloroform (exothermic reaction). The reaction mixture is stirred over night at room temperature and then alkalized with about 25 parts sodium hydroxide 20% at a temperature of 20°C. The aqueous layer is separated and extracted twice with chloroform. The combined organic layers are washed with water, dried over magnesium sulfate, filtered and evaporated. The oily residue is dissolved in a mixture of 40 parts acetone and 20 parts diisopropyl ether and evaporated again. The solid residue is triturated in diisopropylether, yielding DL-4-(N-acetylaminomethyl)-1-[2-(1,4- benzodioxanyl)-methyl]-4-phenylpiperidine; melting point 140°-141.1°C, as a white microcrystalline powder.
From 4.1 parts DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4- phenylpiperidine dihydrochloride, the free base is liberated in the usual manner and extracted with chloroform. The organic layer is separated, dried and evaporated. The DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4- phenylpiperidine obtained is dissolved in 128 parts anhydrous chloroform. This solution is cooled to 5°C and there is added dropwise a solution of 1.6 parts acetylchloride in 7 parts anhydrous chloroform (exothermic reaction). The reaction mixture is stirred over night at room temperature and then alkalized with about 25 parts sodium hydroxide 20% at a temperature of 20°C. The aqueous layer is separated and extracted twice with chloroform. The combined organic layers are washed with water, dried over magnesium sulfate, filtered and evaporated. The oily residue is dissolved in a mixture of 40 parts acetone and 20 parts diisopropyl ether and evaporated again. The solid residue is triturated in diisopropylether, yielding DL-4-(N-acetylaminomethyl)-1-[2-(1,4- benzodioxanyl)-methyl]-4-phenylpiperidine; melting point 140°-141.1°C, as a white microcrystalline powder.
Preparation Products And Raw materials
Raw materials
Related Product Information
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine