The phenolic resins most widely used are low
molecular weight butylated resols, which contain phenolic
hydroxyl groups and etherified and unetherified methylol groups. The epoxy
resins used have a molecular weight of 3000-4000 and therefore contain
secondary hydroxyl groups.
Phenol-formaldehyde resin is used in binders, adhesives, laminates, impregnation products, surface coatings, casting sand, etc.
Phenol-formaldehyde resin is prepared as follows:
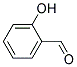
C6H5OH+H2C=O ---> [-C6H2(OH)CH2-]n
One-Stage Resins. The ratio of formaldehyde to phenol is high enough to allow the thermosetting process to take place without the addition of other sources of cross-links.
Two-Stage Resins. The ratio of formaldehyde to phenol is low enough to prevent the thermosetting reaction from occurring during manufacture of the resin. At this point the resin is termed novolac resin. Subsequently, hexamethylenetetramine is incorporated into the material to act as a source of chemical cross-links during the molding operation (and conversion to the thermoset or cured state).
Flammability and Explosibility
Not classified
Phenol-formaldehyde resin is a synthetic resin, commonly known as phenolic, made by the reaction of phenol and formaldehyde, and employed as a molding material for the making of mechanical and electrical parts. The resins are also used for laminating, coatings, and casting resins.
Phenolic resins are used most extensively as thermosetting plastic materials, as there are only a few uses as thermoplastics. The polymer is composed of carbon, hydrogen, oxygen, and sometimes nitrogen. Its molecular weight varies from a very low value during its early state of formation to almost infinity in its final state of cure. The chemical configuration, in the thermoset state, is usually represented by a threedimensional network in which the phenolic nuclei are linked by methylene groups. The completely cross-linked network requires three methylene groups to two phenolic groups. A lesser degree of cross-linking is attainable either by varying the proportions of the ingredients or by blocking some of the reactive positions of the phenolic nucleus by other groups, such as methyl, butyl, etc. Reactivity can be enhanced by increasing the hydroxyl groups on the phenolic nuclei, for example, by the use of resorcinol.
The outstanding characteristics of phenolics are good electrical properties, very rigid set, good tensile strength, excellent heat resistance, good rigidity at elevated temperature, good aging properties; also, good resistance to water, organic solvents, weak bases, and weak acids. All these characteristics are coupled with relatively low cost.
Phenolic resins are used for low-cost partsrequiring good electrical insulating properties,heat resistance, or chemical resistance. Theaverage shelf life of this resin is about 1 monthat 21.1°C. This can be extended by storing it ina refrigerator at 1.6 to 10°C. Varying the catalyst(according to the thickness of the cast) andraising the cure temperature to 93°C will alterthe cure time from as long as 8 h to as short as15 min.
Some shrinkage occurs in the finished casting(0.012 to 0.6 mm/mm), depending on thequantity of filler, amount of catalyst, and therate of cure. Faster cure cycles produce a higherrate of shrinkage. Since the cure cycle can beaccelerated, phenolics are used in short-runcasting operations.
Cast phenolic parts are easily removed fromthe mold if the parting agents recommended bythe supplier are used. Posteuring improves thebasic properties of the finished casting.