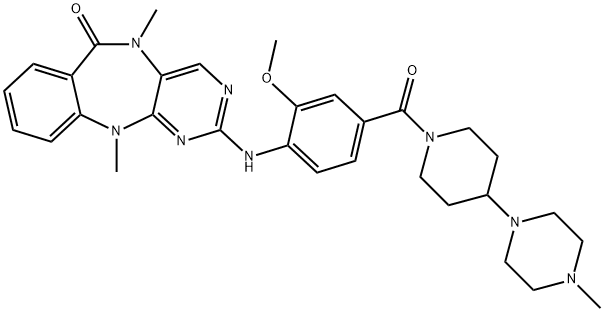
Leucine-rich repeat kinase 2 (LRRK2) is an enzyme that interacts with parkin, a ligase that is part of the ubiquitin-proteasome system that mediates the targeting of proteins for degradation. Loss of function of the parkin protein leads to dopaminergic cell death. The development of Parkinson''s disease has been strongly associated with mutations in the LRRK2 gene that lead to increased kinase activity. LRRK2-IN-1 is a potent inhibitor of LRRK2 that inhibits both wild-type and G2019S mutant LRRK2 (IC50s = 13 and 6 nM, respectively). It is selective for LRRK2 over a large panel of other kinases. LRRK2-IN-1 treatment causes dephosphorylation of LRRK2, leading to its dissociation from 14-3-3 proteins, ubiquitination, and degradation. Inhibitors of LRRK2, including LRRK2-IN-1, stimulate macroautophagy in H4 neuroglioma cells.
LRRK2-IN-1 is a benzodiazepine based derivative and is known as a selective inhibitor of the Parkinson''s disease kinase LRRK2.
ChEBI: LRRK2-IN-1 is a member of the class of pyrimidobenzodiazepines that is 5,11-dimethylpyrimido[4,5-b][1,4]benzodiazepin-6-one carrying at C-2 on the pyrimidine ring a 4-[(4-methylpiperazin-1-yl)piperidine-1-carbonyl]-2-methoxyanilino substituent. It is an inhibitor of the Parkinson's disease kinase LRRK2. It has a role as an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor and an antineoplastic agent. It is a N-acylpiperidine, a N-alkylpiperazine, an aromatic ether, a pyrimidobenzodiazepine, an aromatic amine, a secondary amino compound and a tertiary amino compound.