Standard
In the analysis work, it often demanded to apply high-quality compound with the exact content being accurate in order to be compared as the detection control or used to calibrate the instrument, the compound is known as standard sample.
Standard refers to the standard substance used for measuring the potency of biological products. When the production department submits to a higher authority for approval of the quality standard of the biological product, they should also provide the standard sample (indicated in unit (μ)) used for measuring the potency of the tested product.
According to different application, the verification products can be divided into biological standard for the testing of biological products, endocrine drugs, antibiotics and other bioassay and chemical standards including:
1 chemical standards used for calibration of the instrument;
2 chemical reference standards used for calibration of the ultraviolet or infrared spectrum of the drugs;
3 control standards used for the purity test of drugs as well as the impurity control or as the control standards for content determination of the medicine.
In the page 67 of the appendix in the People's Republic of China Pharmacopoeia 1990 1st edition, we can find hundreds kinds of standards including bornyl ester acetate, eugenol and panaxadiol as well as 39 kinds of control medicine for the purify inspection of traditional Chinese medicine, impurity control or the content determination.
International standards
This refers to standard sample, with the potency units of the existing international standard sample potency units as baseline, undergoing further calibration of its potency units on the basis of a wide range of international cooperation. Its potency is expressed in 1U. For a certain product, for the establishment of the first international standard sample, the determination of its potency units should be consulted and decided by the Committee of Experts before the announcement. The international standard is a standard reference substance for worldwide preparation of national standards, so that the international standards can achieve unity.
Reagents and standards
All reagents in the food analysis laboratories should be clearly labeled to indicate the following: 1 name; 2 the date of preparation; 3 Signature from the people in charge; 4 if necessary, we need to add content such as safety and validity and so on.
Most food analysis should use standard sample as reference for qualitative and quantitative determination. The standard sample should be classified according to the following classification methods: 1 international standard; 2 national standards; 3 internal standard; 4 other standards.
Receptacle for reference standards should be clearly affixed to the label, to indicate the following: 1 name; 2 standard document number; 3 Opening times; 4 Validity; 5 Signature.
In addition, the laboratory should keep all the necessary specific files for all the necessary reference standards.
Capacity analysis standard solution should also be strictly managed. It should be clearly posted on all the standard solution containers with labels to indicate: 1 name; 2 concentration; 3 formulation date; 4 No of formulated procedures; 5 storage conditions; 6 validity; 7 Signature from people in charge.
- Chemical Name:Melphalan IMpurity
- CAS:
- MF:
- Structure:
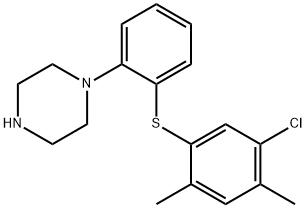
- Chemical Name:1-[2-[(5-chloro-2,4-diMethylphenyl)thio]phenyl]- Piperazine
- CAS:1240670-87-4
- MF:C18H21ClN2S
- Structure:
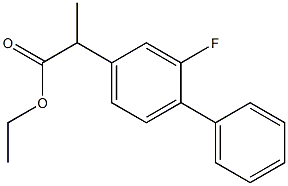
- Chemical Name:Flurbiprofen Impurity
- CAS:64858-90-8
- MF:C17H17FO2
- Structure:
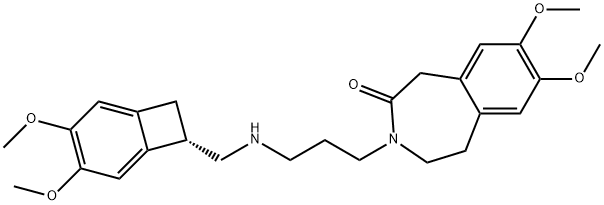
- Chemical Name:the Metabolite of Ivabradine
- CAS:215935-23-2
- MF:C26H34N2O5
- Structure:
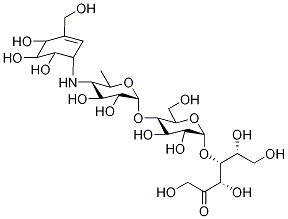
- Chemical Name:Acarbose 1,1-α,α-Glycoside IMpurity
- CAS:610271-07-3
- MF:C25H43NO18
- Structure:

- Chemical Name:Acarbose 1,1-α,α-Glycoside IMpurity
- CAS:610271-07-3
- MF:C25H43NO18
- Structure:
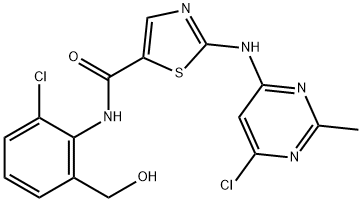
- Chemical Name:Des-6-[4-(2-hydroxyethyl)-1-piperazinyl]-6-chloro Dasatinib
- CAS:910297-71-1
- MF:C16H13Cl2N5O2S
- Structure:
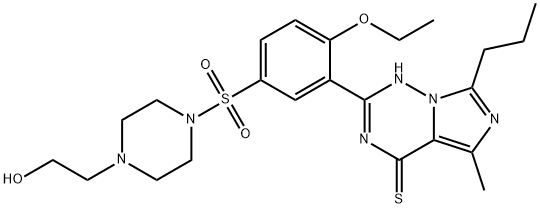
- Chemical Name:Hydroxythiovardenafil
- CAS:912576-30-8
- MF:C23H32N6O4S2
- Structure:
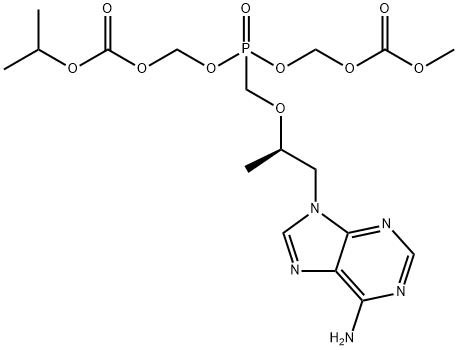
- Chemical Name:(((((1-(6-aMino-9H-purin-9-yl)propan-2-yl)oxy)Methyl)phosphoryl)bis(oxy))bis(Methylene) isopropyl Methyl dicarbonate
- CAS:1246812-43-0
- MF:C17H26N5O10P
- Structure:
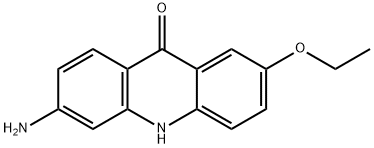
- Chemical Name:9(10H)-Acridinone, 6-aMino-2-ethoxy-
- CAS:144335-20-6
- MF:C15H14N2O2
- Chemical Name:parecoxib impurity L
- CAS:
- MF:
- Structure:
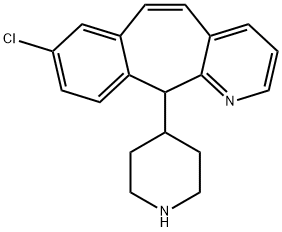
- Chemical Name:Rupatadine iMpurity
- CAS:169253-15-0
- MF:C19H19ClN2
- Chemical Name:ceftazidiMe iMpurity C
- CAS:
- MF:
- Structure:
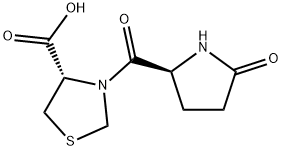
- Chemical Name:pidotiMod iMpurity E
- CAS:162148-16-5
- MF:C9H12N2O4S
- Structure:
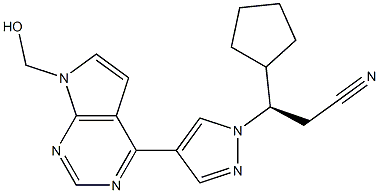
- Chemical Name:1H-Pyrazole-1-propanenitrile, β-cyclopentyl-4-[7-(hydroxyMethyl)-7H-pyrrolo[2,3-d]pyriMidin-4-yl]-,(βR)-
- CAS:1236033-03-6
- MF:C18H20N6O
- Structure:
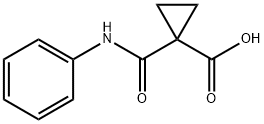
- Chemical Name:Cyclopropanecarboxylic acid, 1-[(phenylaMino)carbonyl]-
- CAS:145591-80-6
- MF:C11H11NO3
- Structure:
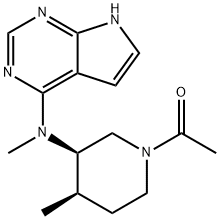
- Chemical Name:3-PiperidinaMine, 1-acetyl-N,4-diMethyl-N-1H-pyrrolo[2,3-d]pyriMidin-4-yl-, (3R,4R)- (9CI)
- CAS:477600-76-3
- MF:C15H21N5O
- Structure:
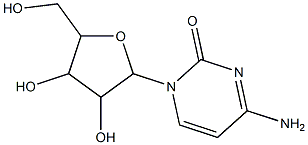
- Chemical Name:Nsc249004
- CAS:7428-39-9
- MF:C9H13N3O5
- Structure:
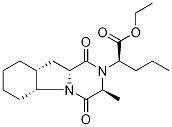
- Chemical Name:(S)-Ethyl 2-((3S,5aS,9aS,10aS)-3-methyl-1,4-dioxodecahydropyrazino[1,2-a]indol-2(1H)-yl)pentanoate ,95%
- CAS:129970-98-5
- MF:C19H30N2O4
- Chemical Name:Exemestane Impurity A
- CAS:
- MF:
- Structure:
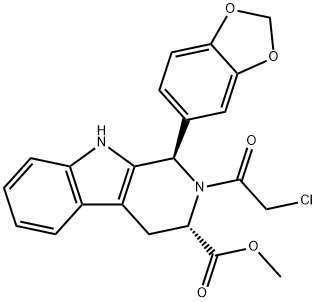
- Chemical Name:(1R,3S)-1-(1,3-Benzodioxol-5-yl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic Acid Methyl Ester
- CAS:629652-44-4
- MF:C22H19ClN2O5
- Structure:
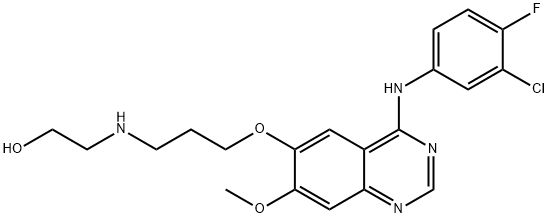
- Chemical Name:3-DesMorpholinyl-3-hydroxyethylaMino Gefitinib
- CAS:847949-56-8
- MF:C20H22ClFN4O3
- Structure:
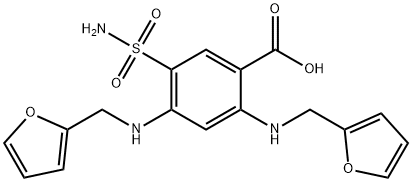
- Chemical Name:4-Deschloro-4-(2-furanylMethyl)aMino FuroseMide
- CAS:5046-19-5
- MF:C17H17N3O6S
- Structure:
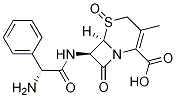
- Chemical Name:Cephalexin Sulfoxide
- CAS:56193-21-6
- MF:C16H17N3O5S
- Structure:
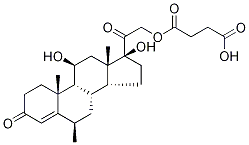
- Chemical Name:6α-Methyl Hydrocortisone 21-HeMisuccinate
- CAS:119657-85-1
- MF:C26H36O8
- Structure:
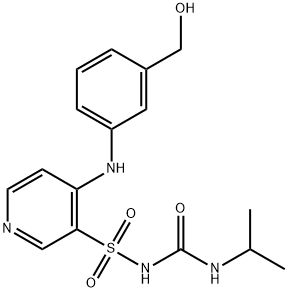
- Chemical Name:Hydroxy TorseMide
- CAS:99300-68-2
- MF:C16H20N4O4S
- Structure:
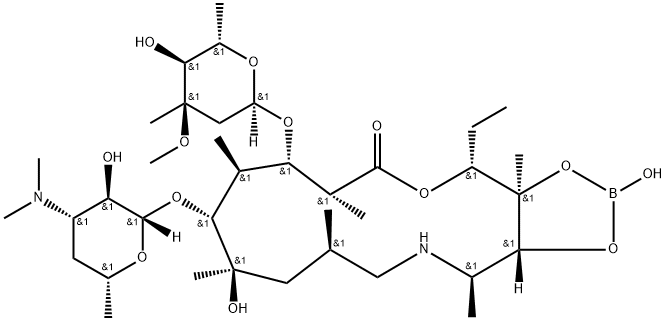
- Chemical Name:AzithroMycin iMpurity
- CAS:194809-66-0
- MF:C37H69BN2O13
- Structure:
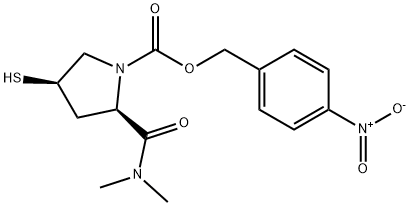
- Chemical Name:MeropeneM iMpurity
- CAS:96035-23-3
- MF:C15H19N3O5S
- Structure:
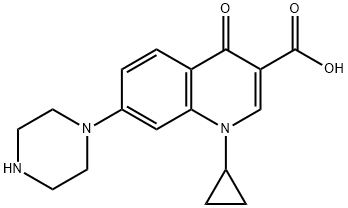
- Chemical Name:Ciprofloxacin EP IMpurity B
- CAS:93107-11-0
- MF:C17H19N3O3
- Structure:
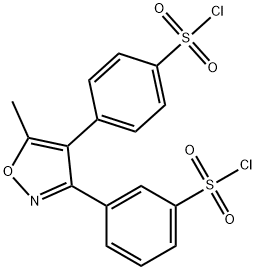
- Chemical Name:Valdecoxib IMpurity-E
- CAS:1373038-63-1
- MF:C16H11Cl2NO5S2
- Structure:
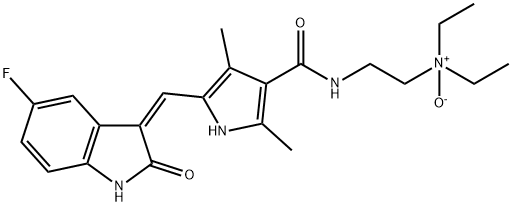
- Chemical Name:Sunitinib N-Oxide
- CAS:356068-99-0
- MF:C22H27FN4O3
- Structure:

- Chemical Name:Abiraterone Sulfate
- CAS:
- MF:
- Chemical Name:AtracuriuM IMpurity G (R-Laudanosine)
- CAS:
- MF:
- Chemical Name:BendaMustine IMpurity D BendaMustine N-Alkylated IMpurity
- CAS:
- MF:
- Chemical Name:Cefradine IMpurity E
- CAS:
- MF:
- Structure:
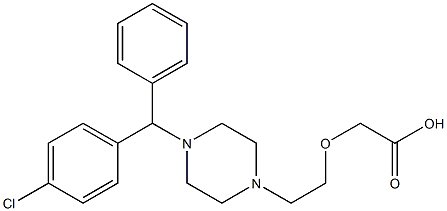
- Chemical Name:Cetirizine IMpurity-7
- CAS:
- MF:C21H25ClN2O3
- Chemical Name:Dasatinib IMpurity 6
- CAS:
- MF:
- Structure:
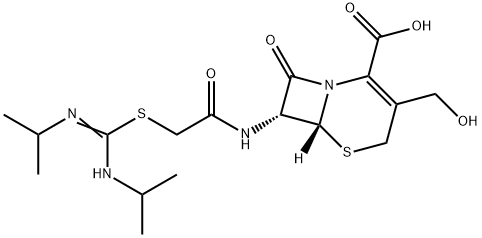
- Chemical Name:Desacetyl CefathiaMidine
- CAS:958001-61-1
- MF:C17H26N4O5S2
- Structure:
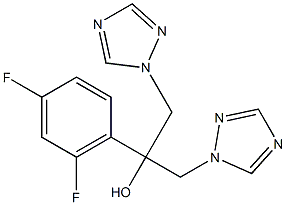
- Chemical Name:Fluconazole IMpurity D
- CAS:
- MF:C13H12F2N6O
- Structure:
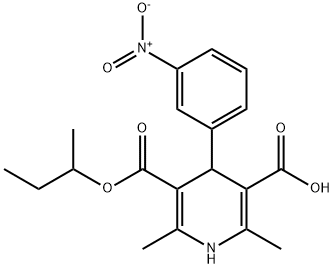
- Chemical Name:Lercanidipine IMpurity A
- CAS:74936-74-6
- MF:C19H22N2O6
- Structure:
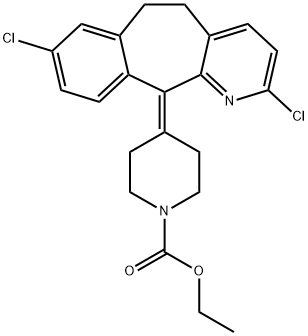
- Chemical Name:Loratadine 2-Chloro IMpurity
- CAS:165739-64-0
- MF:C22H22Cl2N2O2
- Structure:
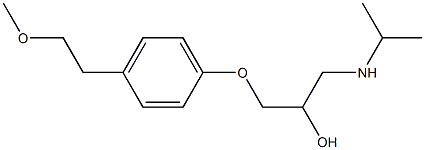
- Chemical Name:Metoprolol IMpurity F
- CAS:
- MF:C15H25NO3
- Structure:
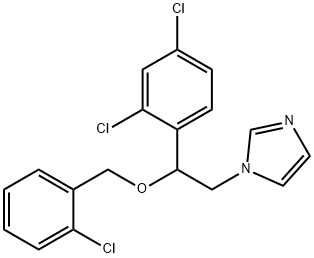
- Chemical Name:Miconazole IMpurity I
- CAS:47363-37-1
- MF:C18H15Cl3N2O
- Chemical Name:Milrinone IMpurity 1
- CAS:
- MF:
- Structure:
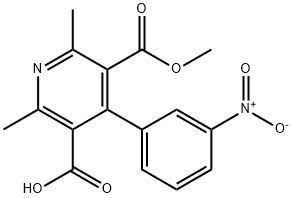
- Chemical Name:Nicardipine Related CoMpound 2
- CAS:64603-72-1
- MF:C16H14N2O6
- Structure:
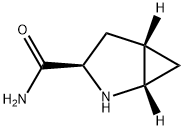
- Chemical Name:Saxagliptin IMpurity 1
- CAS:1564266-73-4
- MF:C6H10N2O
- Structure:
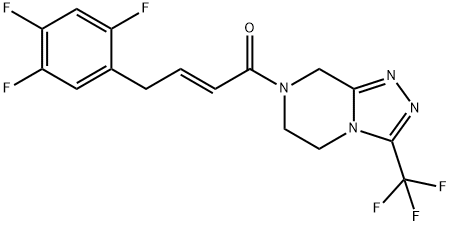
- Chemical Name:Sitagliptin DeaMino IMpurity 1
- CAS:1253056-18-6
- MF:C16H12F6N4O
- Structure:
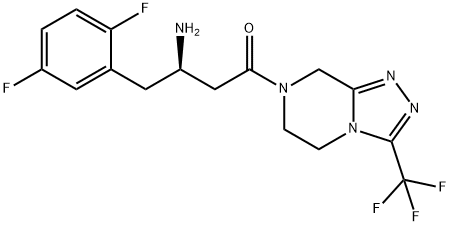
- Chemical Name:Sitagliptin Defuoro IMpurity 5
- CAS:486460-31-5
- MF:C16H16F5N5O
- Structure:
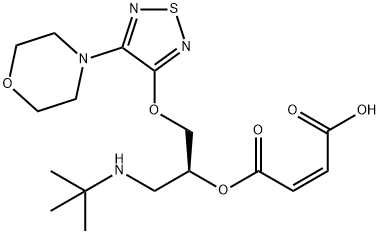
- Chemical Name:TiMolol IMpurity E
- CAS:1026075-53-5
- MF:C17H26N4O6S
- Structure:
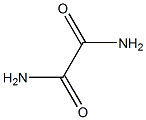
- Chemical Name:EthanediaMide iMpurity J
- CAS:
- MF:C2H4N2O2
- Structure:
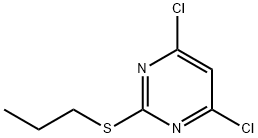
- Chemical Name:4,6-dichloro-2-(propylthio)pyriMidine
- CAS:1401318-10-2
- MF:C7H8Cl2N2S
- Chemical Name:Istradefylline impurity B
- CAS:
- MF:
- Structure:
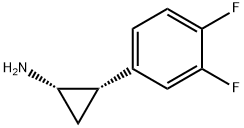
- Chemical Name:Ticagrelor diastereoMer
- CAS:1459719-81-3
- MF:C9H9F2N
- Structure:
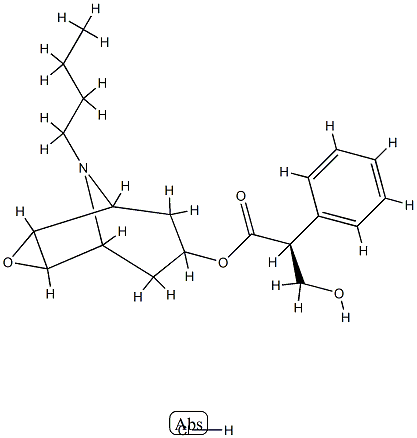
- Chemical Name:N-Butyl Nor ScopolaMine Hydrochloride
- CAS:22235-98-9
- MF:C20H28ClNO4
- Structure:
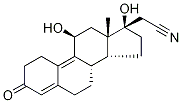
- Chemical Name:11β-Hydroxy Dienogest
- CAS:86153-39-1
- MF:C20H25NO3
- Structure:
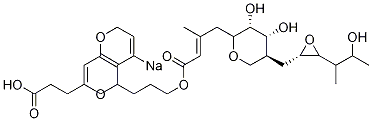
- Chemical Name:2H,5H-Pyrano[4,3-b]pyranyl Mupirocin SodiuM IMpurity
- CAS:116182-44-6
- MF:C26H43NaO9
- Structure:
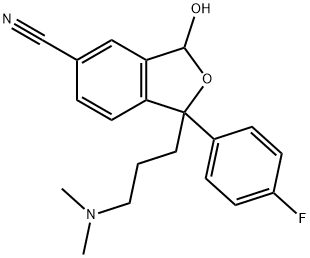
- Chemical Name:3-Hydroxy CitalopraM
- CAS:411221-53-9
- MF:C20H21FN2O2
- Structure:
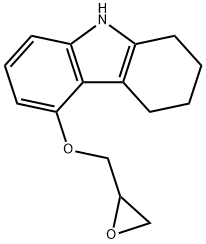
- Chemical Name:5-(OxiranylMethoxy)-2,3,4,9-tetrahydrocarbazole
- CAS:58457-32-2
- MF:C15H17NO2
- Structure:
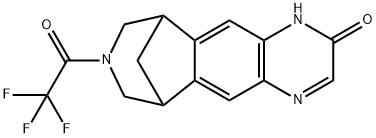
- Chemical Name:Hydroxy Varenicline N-Trifluoroacetate
- CAS:357426-10-9
- MF:C15H12F3N3O2
- Structure:
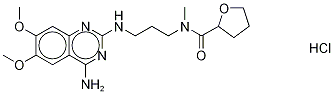
- Chemical Name:N2-Methyl Alfuzosin Hydrochloride
- CAS:72104-34-8
- MF:C19H28ClN5O4
- Structure:

- Chemical Name:N2-Methyl Alfuzosin Hydrochloride
- CAS:72104-34-8
- MF:C19H28ClN5O4
- Structure:
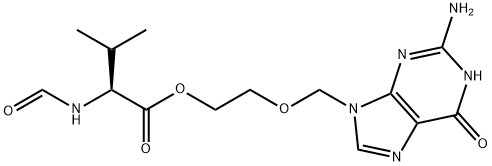
- Chemical Name:N-ForMyl Valacyclovir
- CAS:847670-62-6
- MF:C14H20N6O5
- Structure:
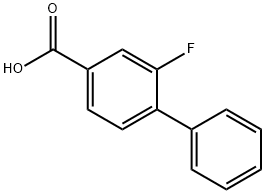
- Chemical Name:2-Fluorobiphenyl-4-carboxylic Acid
- CAS:137045-30-8
- MF:C13H9FO2
- Structure:
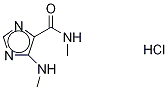
- Chemical Name:Theophyllidine Hydrochloride
- CAS:116131-08-9
- MF:C6H10ClN4O
- Structure:
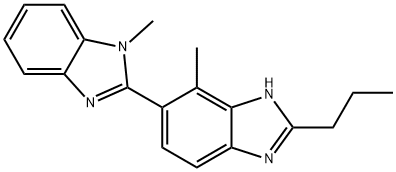
- Chemical Name:1,4'-Dimethyl-2'-propyl-2,5'-bi-1H-benzimidazole
- CAS:887583-89-3
- MF:C19H20N4
- Chemical Name:Tetracyclics compound impurities
- CAS:
- MF:
- Structure:
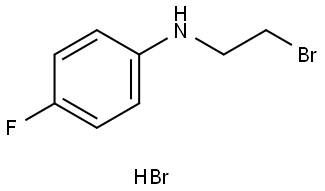
- Chemical Name:Urapidil Impurity
- CAS:770-42-3
- MF:C8H9BrFN.BrH
- Structure:
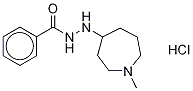
- Chemical Name:N'-(1-Methylazepan-4-yl)benzohydrazine
- CAS:117078-69-0
- MF:C14H22ClN3O
- Structure:
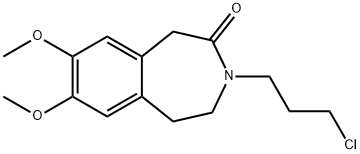
- Chemical Name:3-(3-Chloropropyl)-7,8-dimethoxy-2,3,4,5-tetrahydro-1H-3-benzazepin-2-one
- CAS:85175-65-1
- MF:C15H20ClNO3
- Structure:
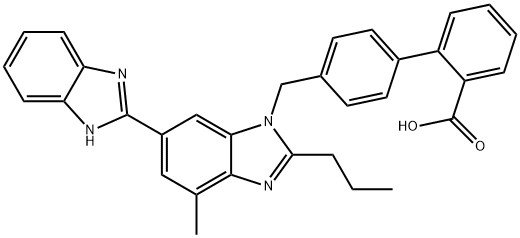
- Chemical Name:N-DesMethyl TelMisartan
- CAS:144701-81-5
- MF:C32H28N4O2
- Structure:
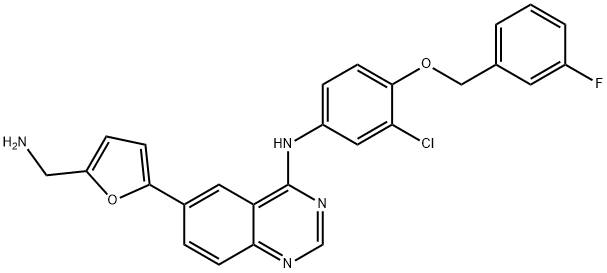
- Chemical Name:N-De[2-(Methylsulfonyl)ethyl] Lapatinib
- CAS:697299-82-4
- MF:C26H20ClFN4O2
- Structure:
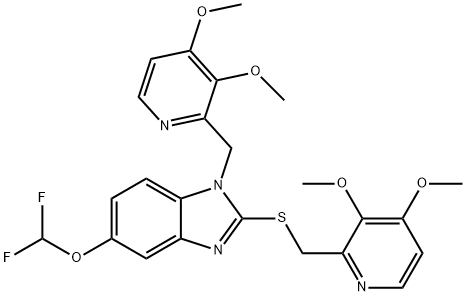
- Chemical Name:N-[(3,4-DiMethoxy-2-pyridinyl)Methyl] Pantoprazole Sulfide
- CAS:957470-58-5
- MF:C24H24F2N4O5S
- Structure:
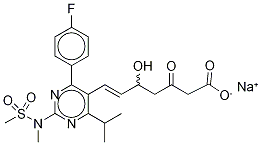
- Chemical Name:3-Oxo Rosuvastatin SodiuM Salt
- CAS:1346606-28-7
- MF:C22H25FN3NaO6S
- Structure:
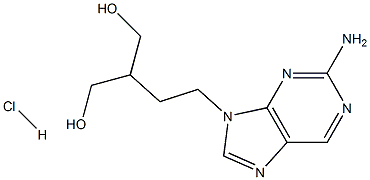
- Chemical Name:Famciclovir Related Compound A (20 mg) (2-[2-(2-Amino-9H-purin-9-yl)ethyl]propane-1,3-diol hydrochloride)
- CAS:246021-75-0
- MF:C10H16ClN5O2
- Structure:
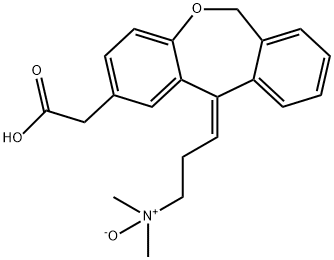
- Chemical Name:Olopatadine USP RC B
- CAS:173174-07-7
- MF:C21H23NO4
- Structure:
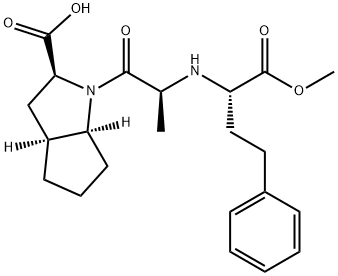
- Chemical Name:Ramipril Related Compound A (30 mg) ((2S,3aS,6aS)-1-[(S)2-[[(S)-1-(methoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-octahydrocyclopenta[b]pyrrole-2-carboxylic acid)
- CAS:108313-11-7
- MF:C22H30N2O5
- Structure:
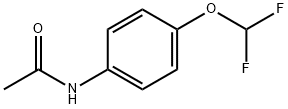
- Chemical Name:4'-(Difluoromethoxy)acetanilide
- CAS:22236-11-9
- MF:C9H9F2NO2
- Structure:
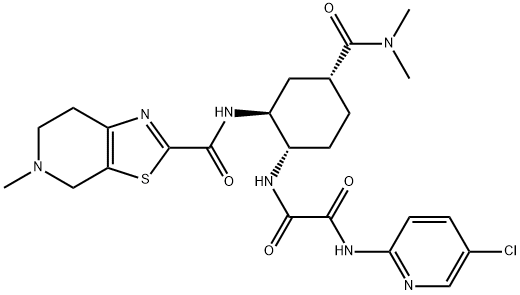
- Chemical Name:EthanediaMide, N1-(5-chloro-2-pyridinyl)-N2-[(1S,2S,4R)-4-[(diMethylaMino)carbonyl]-2-[[(4,5,6,7-tetrahydro-5-Methylthiazolo[5,4-c]pyridin-2-yl)carbonyl]aMino]cyclohexyl]-
- CAS:1255529-28-2
- MF:C24H30ClN7O4S
- Structure:
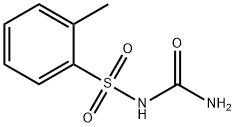
- Chemical Name:N-carbaMoyl-2-Methyl benzene sulfonaMide
- CAS:39051-77-9
- MF:C8H10N2O3S
- Structure:
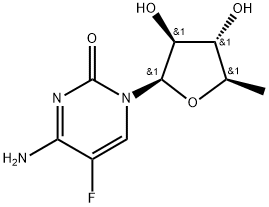
- Chemical Name:Capecitabin iMpurity
- CAS:71609-08-0
- MF:C9H12FN3O4
- Chemical Name:Ibuprofen IMp. I (EP): 1-(2-Methylpropyl)-4-[(3RS)-3-[4-(2-Methylpropyl)phenyl]butyl]benzene
- CAS:
- MF:
- Structure:
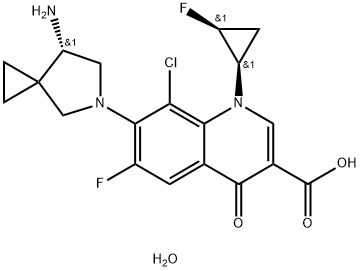
- Chemical Name:3-Quinolinecarboxylic acid, 7-[(7S)-7-aMino-5-azaspiro[2.4]hept-5-yl]-8-chloro-6-fluoro-1-[(1R,2S)-2-fluorocyclopropyl]-1,4-dihydro-4-oxo-, hydrate
- CAS:163253-37-0
- MF:C19H20ClF2N3O4
- Structure:
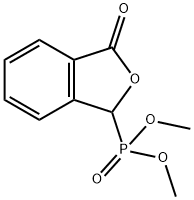
- Chemical Name:3-oxo-1,3-dihydroisobenzofuran-1-ylphosphonic acid
- CAS:61260-15-9
- MF:C10H11O5P
- Structure:
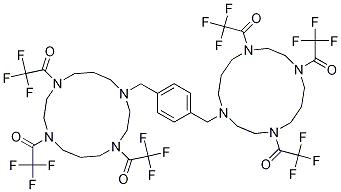
- Chemical Name:1-(4,8-bis-(2,2,2-trifluoroacetyl)-11-{4-[4,8,11-tris-(2,2,2-trifluoroacetyl)-1,4,8,11-tetraazacyclotetradec-1-ylMethyl]-benzyl}-1,4,8,11-tetraazacyclotetradec-1-yl)-2,2,2-trifluoroethanone
- CAS:406939-93-3
- MF:C40H48F18N8O6
- Chemical Name:Anastrozole iMpurity A
- CAS:
- MF:
- Chemical Name:Anastrozole iMpurity A
- CAS:
- MF:
- Chemical Name:EMtricitabine iMpurity C
- CAS:
- MF:
- Chemical Name:IMp. F (EP)
- CAS:
- MF:
- Chemical Name:RocuroniuMbroMide iMpurity F
- CAS:
- MF:
- Structure:
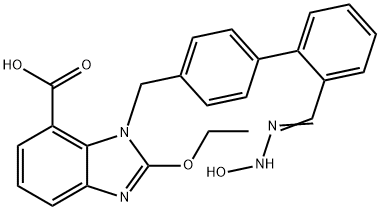
- Chemical Name:(Z)-2-ethoxy-3-((2'-(N'-hydroxycarbaMiMidoyl)biphenyl-4-yl)Methyl)-3H-benzo[d]iMidazole-4-carboxylic acid
- CAS:1397836-49-5
- MF:C24H22N4O4
- Structure:
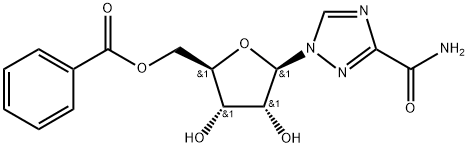
- Chemical Name:Ribavirin IMpurity E
- CAS:58151-90-9
- MF:C15H16N4O6
- Structure:
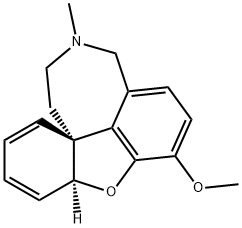
- Chemical Name:TetrahydrogalantaMine
- CAS:664995-65-7
- MF:C17H19NO2
- Structure:
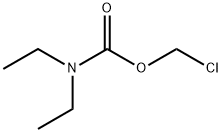
- Chemical Name:Diethyl-carbaMic Acid ChloroMethyl Ester
- CAS:133217-92-2
- MF:C6H12ClNO2
- Structure:
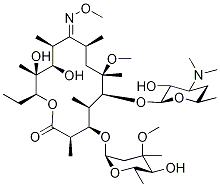
- Chemical Name:ClarithroMycin IMpurity G
- CAS:127182-44-9
- MF:C39H72N2O13
- Structure:
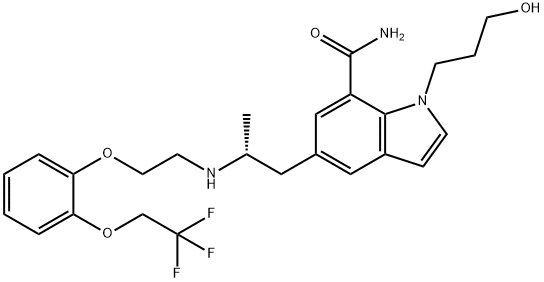
- Chemical Name:1-(3-Hydroxypropyl)-5-[(2R)-2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]aMino]propyl]-1H-indole-7-carboxaMide
- CAS:175870-21-0
- MF:C25H30F3N3O4
- Structure:
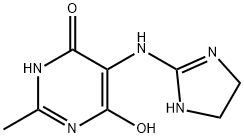
- Chemical Name:5-[(4,5-Dihydro-1H-iMidazol-2-yl)aMino]-6-hydroxy-2-Methyl-4(3H)-pyriMidinone
- CAS:352457-32-0
- MF:C8H11N5O2
- Structure:
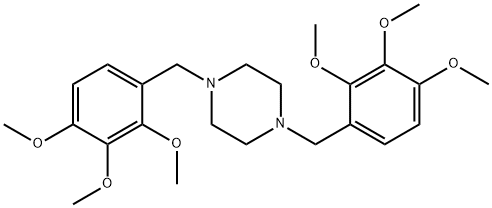
- Chemical Name:1,4-Bis(2,3,4-triMethoxybenzyl)piperazine
- CAS:1257-19-8
- MF:C24H34N2O6
- Structure:
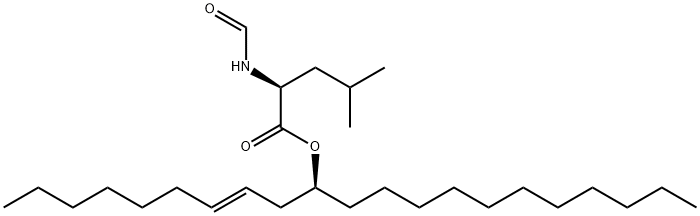
- Chemical Name:N-ForMyl-L-leucine [S-(E)]-1-(2-Nonenyl)dodecyl Ester
- CAS:130676-63-0
- MF:C28H53NO3