Terpenes
Terpenes is the generic term summarizing all kinds of isoprene polymers and their derivatives with its general formula of (C5H8) n. Terpene is a class of compound that is ubiquitous in the plant kingdom, but exist in very few amount in the animal kingdom. Except in the form of terpene hydrocarbons, there are a large number of terpenes forming various kinds of oxygenated derivatives including alcohols, aldehydes, ketones, carboxylic acids, esters and glycoside forms. Secondly there are also nitrogen-containing derivatives as well as a minority of sulfur-containing derivatives presented. Based on the number of the isoprene units contained inside the molecule, the terpenes can be divided into: monoterpene, sesquiterpenoid, diterpene, sesquiterpene, triterpenoids, tetraterpene and polyterpene.
For some compound which originated from the synthesis of isoprene, but has the number of carbon atoms in the molecule be not an integer multiple of 5, they are called terpenoid. In the life activities, terpenoids compound, especially inside the plants, have important functions, for example gibberellin, abscisic acid and insect juvenile hormone are important hormones, carotenoids and chlorophyll are important photosynthetic pigments; plastoquinone and quinone are respectively important electronic chain delivery body in photosynthesis and respiration chain; sterols are the component of the biological membrane.
Monoterpene and sesquiterpene are the major component of volatile oil. Diterpene is the major substance forming the resin; triterpenoid is an important material forming plant saponins and resins, tetraterpene mainly include some fat-soluble pigments widely distributed in the plants. In nature, terpenoids is widely distributed, some of which have physiological activity. For example, ascaridole and santonin have roundworm expelling effect; artemisinin has antimalarial effect while andrographolidume has antibacterial effect.
The following describes some of the chemical reactions associated with terpenes. This is significant for determination of the chemical structure of terpene component.
(1) Oxidation reaction
Different oxidants, in different situations, can oxidize the different groups contained in terpene component to produce different products. For example, chromic acid can oxidize the carbon methyl group and gem-dimethyl carbon to produce acetic acid; the oxidation reaction of ozone is a valuable double bond cleavage reaction and can determine the position of double bond in the structure of the terpene composition. Lead tetraacetate is also oxidation agents of double-bond and has been widely used in chemical research of the terpene ingredients.
(2) The dehydrogenation
Dehydrogenation reaction can be considered as a kind of oxidation reactions and is a kind of valuable reaction for the study the chemical structure of terpenes particularly cyclic terpene. It refers to that the terpene component is heated (200 ℃ ~ 300 ℃) together with sulfur or selenium under an inert atmosphere, the carbon skeleton of the cyclic terpene is dehydrogenated into aromatic derivatives with sometimes the ring being cleaved and sometimes cyclization occurring simultaneously.
(3) Addition reaction
The double bonds in the terpene components can react with hydrogen halide acids such as hydroiodic acid or hydrogen chloride in glacial acetic acid solution to generate crystalline-shaped addition product. It can also absorb bromine (diethyl ether or glacial acetic acid - ethanol solution) to generate bromide with certain physicochemical properties. If mixing the glacial acetic acid and sodium nitrite for shaking, nitrous oxide or pseudo-nitrous oxide will be generate and will exhibit visible special colors.
If the unsaturated terpene component was added with amyl nitrite and concentrated hydrochloric acid for mixing with shaking and keep it cool, then add a small amount of ethanol or glacial acetic acid, there will be chlorinated nitroso derivative of terpenes generated with special colors as well. Such nitroso derivatives (including nitrous oxides and chlorinated nitroso derivative) mostly exhibit blue or blue-green color. They are easily polymerized to form a colorless di-polymer, but when the di-polymer is heated to a molten state or when made into a solution, it can also be converted into a blue or blue-green single-molecule compound.
Nitroso chlorinated derivatives can condense with primary or secondary amines (commonly piperidine) to generate nitroso amines with most of them having complete crystal shape and certain physical and chemical constants available for the identification of unsaturated terpene components. If the terpene component molecules contain conjugated double bonds, then it will form crystalline addition product with maleic anhydride through Diels-Alder reaction which can be used to prove the presence of conjugated double bonds.
(4) Wagner-Meerweein rearrangement;
Terpene molecules, sometimes through the effect of agents (such as acid, etc.), the carbon skeleton can be changed or the functional groups in the molecule can be transferred in the molecule. Especially during the elimination reaction, addition reaction or a nucleophilic substitution reaction of bicyclic terpene compound, there is often Wagner-Meerweein rearrangement happening.
- Structure:
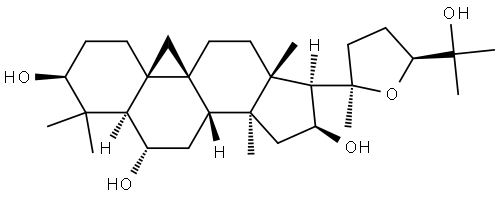
- Chemical Name:Cycloastragenol
- CAS:78574-94-4
- MF:C30H50O5
- Structure:
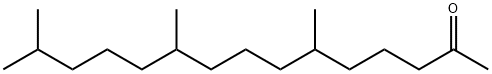
- Chemical Name:FITONE
- CAS:502-69-2
- MF:C18H36O
- Structure:
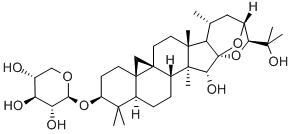
- Chemical Name:CIMIGENOL 3-O-BETA-D-XYLOPYRANOSIDE
- CAS:27994-11-2
- MF:C35H56O9
- Structure:
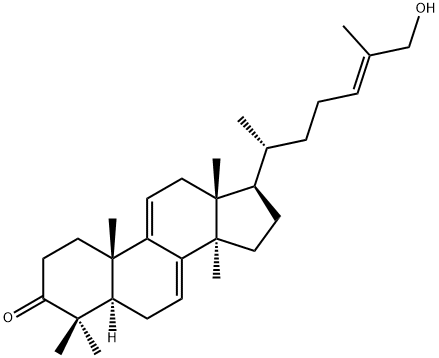
- Chemical Name:Ganoderol A
- CAS:104700-97-2
- MF:C30H46O2
- Structure:
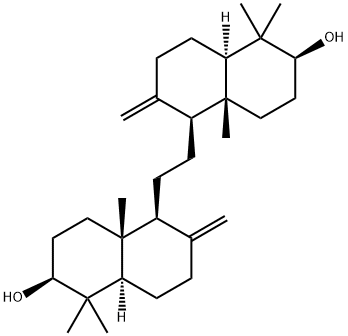
- Chemical Name:ALPHA-ONOCERIN
- CAS:511-01-3
- MF:C30H50O2
- Structure:
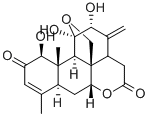
- Chemical Name:ailanthone
- CAS:981-15-7
- MF:C20H24O7
- Structure:
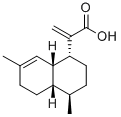
- Chemical Name:artemisic acid
- CAS:80286-58-4
- MF:C15H22O2
- Structure:

- Chemical Name:DESACETYLNIMBIN
- CAS:18609-16-0
- MF:C28H34O8
- Structure:
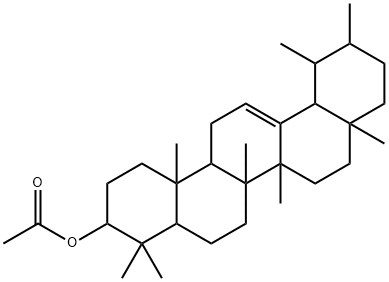
- Chemical Name:alpha-Amyrenyl acetate
- CAS:863-76-3
- MF:C32H52O2
- Structure:
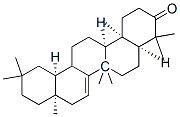
- Chemical Name:(4aS,6aS,6aR,8aR,12aS,14aS,14bS)-4,4,6a,6a,8a,11,11,14b-octamethyl-2,4 a,5,6,8,9,10,12,12a,13,14,14a-dodecahydro-1H-picen-3-one
- CAS:514-07-8
- MF:C30H48O
- Structure:
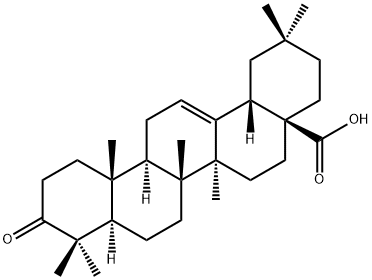
- Chemical Name:3-Oxo-olean-12-en-28-oic acid
- CAS:17990-42-0
- MF:C30H46O3
- Structure:
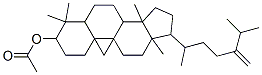
- Chemical Name:1-(4-Isopropyl-1-methyl-4-pentenyl)-3a,6,6,12a-tetramethyltetradecahyd ro-1H-cyclopenta[a]cyclopropa[e]phenanthren-7-yl acetate
- CAS:1259-94-5
- MF:C33H54O2
- Structure:
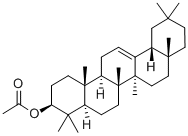
- Chemical Name:[(3S,4aS,6aR,6bS,8aR,12aR,14aS,14bS)-4,4,6a,6b,8a,11,11,14b-octamethyl -1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicen-3-yl] acetate
- CAS:1616-93-9
- MF:C32H52O2
- Structure:
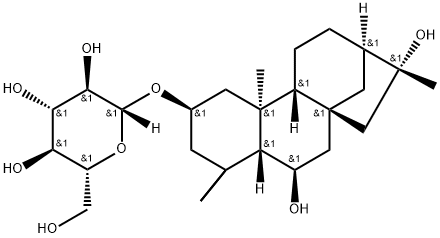
- Chemical Name:6β,16-Dihydroxykauran-2β-yl β-D-glucopyranoside
- CAS:53452-34-9
- MF:C26H44O8
- Structure:
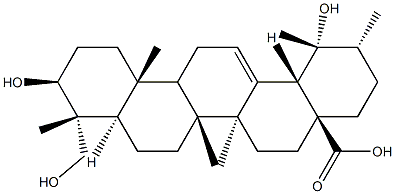
- Chemical Name:Rutundic acid
- CAS:20137-37-5
- MF:C30H48O5
- Structure:
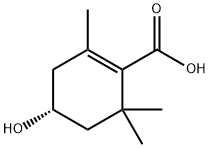
- Chemical Name:4-Hydroxy-2,6,6-trimethyl-1-cyclohexenecarboxylic acid
- CAS:62218-55-7
- MF:C10H16O3
- Structure:
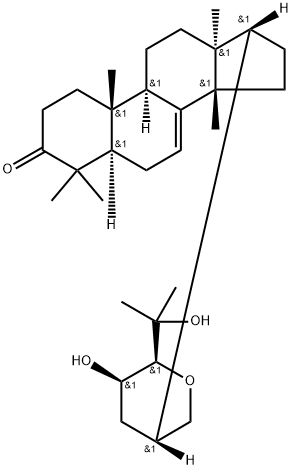
- Chemical Name:(13α,14β,17α,20S,23R,24S)-21,24-Epoxy-23,25-dihydroxy-5α-lanost-7-en-3-one
- CAS:6985-35-9
- MF:C30H48O4
- Structure:
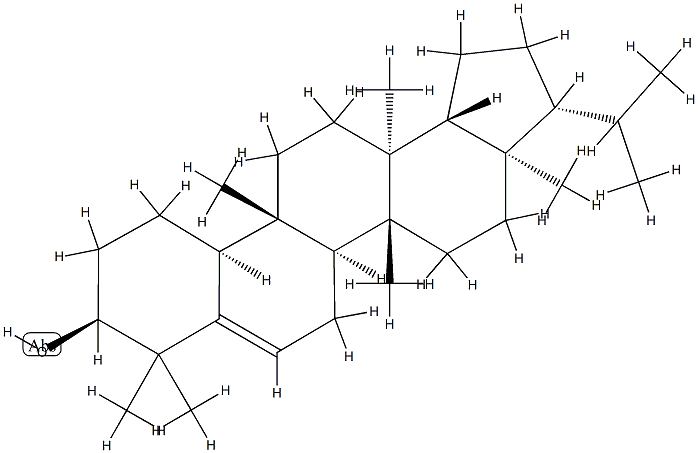
- Chemical Name:D:B-Friedo-B':A'-neogammacer-5-en-3β-ol
- CAS:1615-94-7
- MF:C30H50O
- Structure:
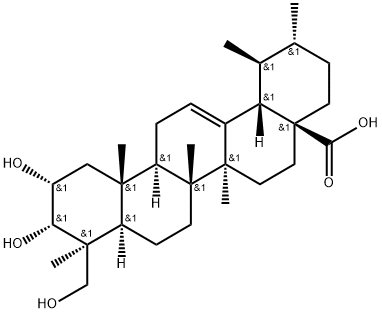
- Chemical Name:2α,3α,24-Trihydroxyurs-12-en-28-oic acid
- CAS:89786-83-4
- MF:C30H48O5
- Structure:
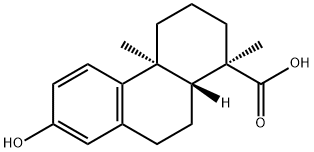
- Chemical Name:13-Hydroxy-8,11,13-podocarpatrien-18-oic acid
- CAS:61597-83-9
- MF:C17H22O3
- Structure:
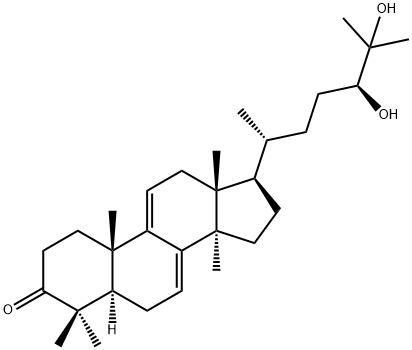
- Chemical Name:Gadermandiol
- CAS:107900-76-5
- MF:C30H48O3
- Structure:
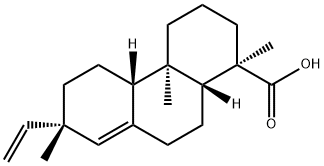
- Chemical Name:PIMARIC ACID
- CAS:127-27-5
- MF:C20H30O2
- Structure:
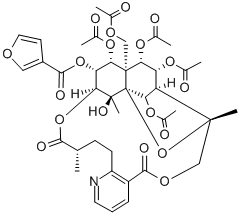
- Chemical Name:WILFORGINE
- CAS:37239-47-7
- MF:C41H47NO19
- Structure:
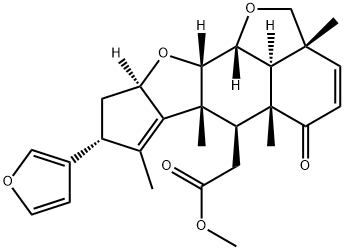
- Chemical Name:28-deoxonimbolide
- CAS:126005-94-5
- MF:C27H32O6
- Structure:
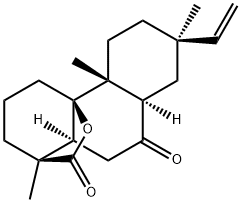
- Chemical Name:rosenonolactone
- CAS:508-71-4
- MF:C20H28O3
- Structure:
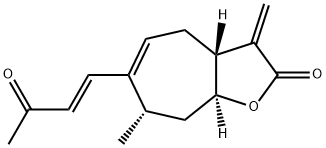
- Chemical Name:xanthatin
- CAS:26791-73-1
- MF:C15H18O3
- Structure:
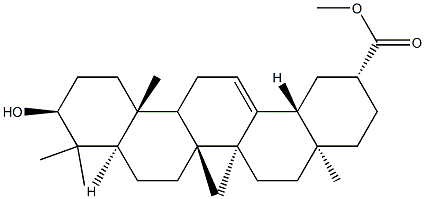
- Chemical Name:3-Epikatonic acid
- CAS:76035-62-6
- MF:C30H48O3
- Structure:
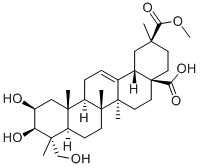
- Chemical Name:Phytolaccagenin
- CAS:1802-12-6
- MF:C31H48O7
- Structure:
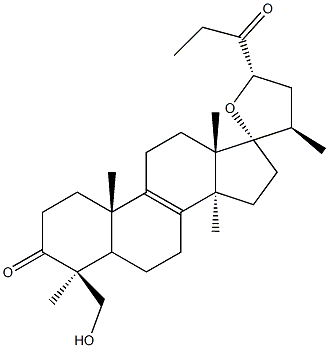
- Chemical Name:(23S)-17,23-Epoxy-29-hydroxy-27-nor-5α-lanost-8-ene-3,24-dione
- CAS:81678-46-8
- MF:C29H44O4
- Structure:
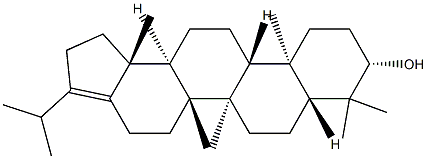
- Chemical Name:Hop-17(21)-en-3β-ol
- CAS:564-14-7
- MF:C30H50O
- Structure:
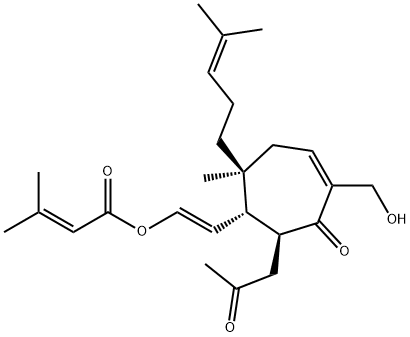
- Chemical Name:3-Methyl-2-butenoic acid [2-[5-hydroxy-2-methyl-2-(4-methyl-3-pentenyl)-6-oxo-7-(2-oxopropyl)-4-cyclohepten-1-yl]vinyl] ester
- CAS:74690-89-4
- MF:C25H36O5
- Structure:
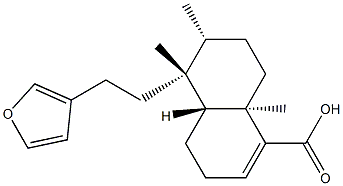
- Chemical Name:(4aR)-5β-[2-(3-Furyl)ethyl]-3,4,4aβ,5,6,7,8,8a-octahydro-5,6α,8aα-trimethyl-1-naphthalenecarboxylic acid
- CAS:1782-65-6
- MF:C20H28O3
- Structure:
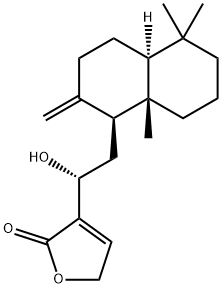
- Chemical Name:12-Hydroxy-8(17),13-labdadien-16,15-olide
- CAS:958885-86-4
- MF:C20H30O3
- Structure:
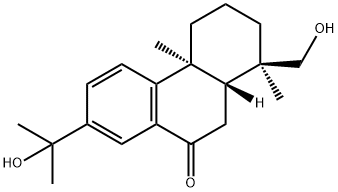
- Chemical Name:15,18-Dihydroxyabieta-8,11,13-trien-7-one
- CAS:213329-45-4
- MF:C20H28O3
- Structure:
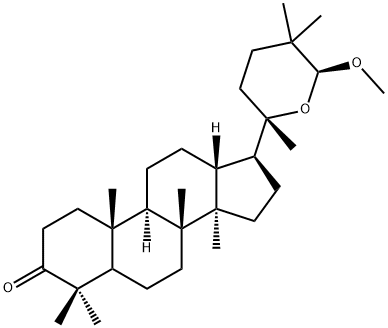
- Chemical Name:20,24-Epoxy-24-methoxy-23(24-25)abeo-dammaran-3-one
- CAS:1020074-97-8
- MF:C31H52O3
- Structure:
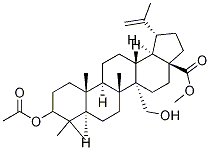
- Chemical Name:3-Acetoxy-27-hydroxy-20(29)-lupen -28-oic acid methyl ester
- CAS:263844-80-0
- MF:C33H52O5
- Structure:
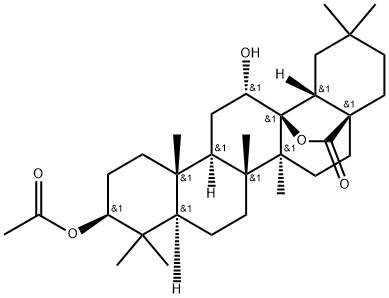
- Chemical Name:3-O-Acetyloleanderolide
- CAS:62498-83-3
- MF:C32H50O5
- Structure:
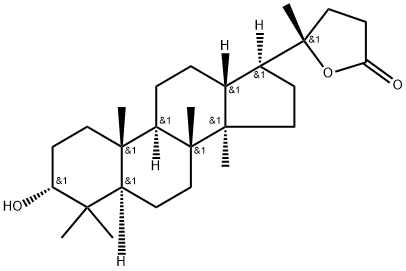
- Chemical Name:Cabraleahydroxylactone
- CAS:35833-69-3
- MF:C27H44O3
- Structure:
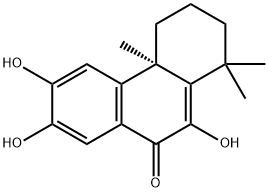
- Chemical Name:Celaphal A
- CAS:244204-40-8
- MF:C17H20O4
- Structure:
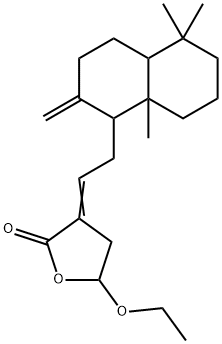
- Chemical Name:Coronarin D ethyl ether
- CAS:138965-89-6
- MF:C22H34O3
- Structure:
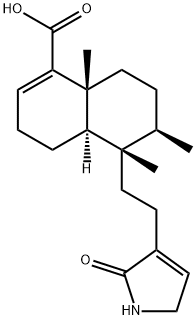
- Chemical Name:Echiphyllin C
- CAS:310433-44-4
- MF:C20H29NO3
- Structure:
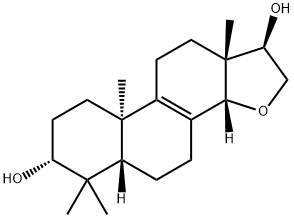
- Chemical Name:ent-14,16-Epoxy-8-pimarene-3,15-diol
- CAS:1188281-98-2
- MF:C20H32O3
- Structure:
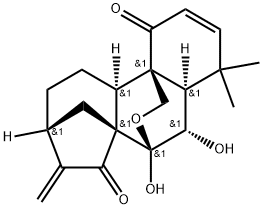
- Chemical Name:Eriocalyxin B
- CAS:84745-95-9
- MF:C20H24O5
- Structure:
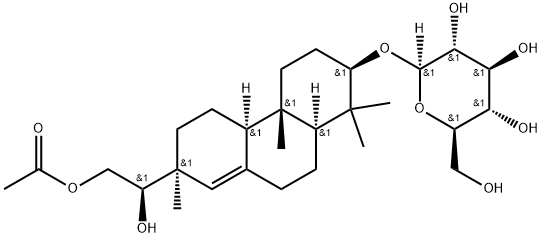
- Chemical Name:Hythiemoside A
- CAS:853267-91-1
- MF:C28H46O9
- Structure:
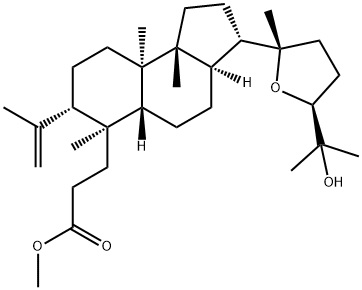
- Chemical Name:Methyl eichlerianate
- CAS:56421-12-6
- MF:C31H52O4
- Structure:
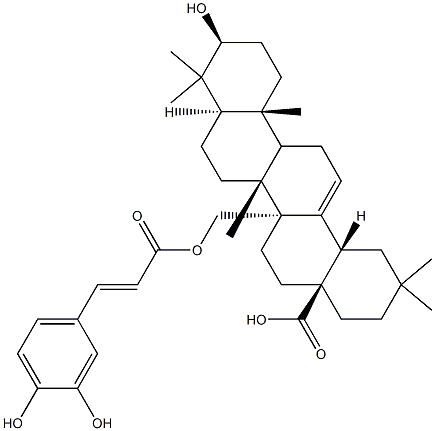
- Chemical Name:Myriceric acid B
- CAS:55497-79-5
- MF:C39H54O7
- Structure:
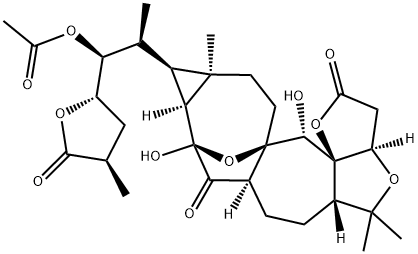
- Chemical Name:Pre-schisanartanin B
- CAS:1033288-92-4
- MF:C31H42O11
- Structure:
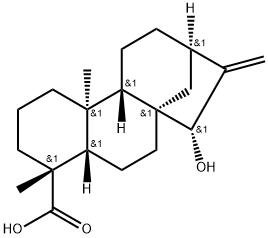
- Chemical Name:grandifloric acid
- CAS:22338-69-8
- MF:C20H30O3
- Structure:
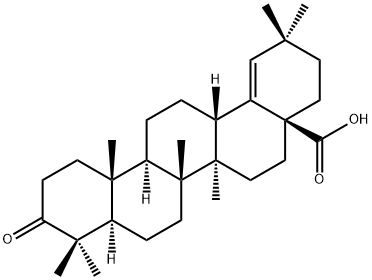
- Chemical Name:moronic acid
- CAS:6713-27-5
- MF:C30H46O3
- Structure:
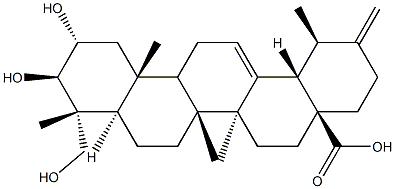
- Chemical Name:actinidic acid
- CAS:341971-45-7
- MF:C30H46O5
- Structure:
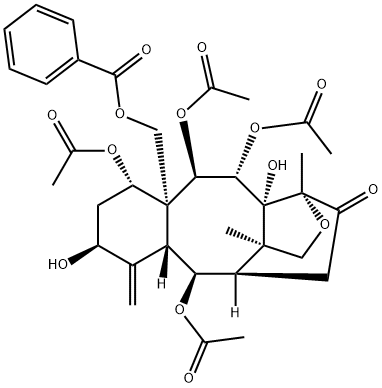
- Chemical Name:taxinine M
- CAS:135730-55-1
- MF:C35H42O14
- Structure:
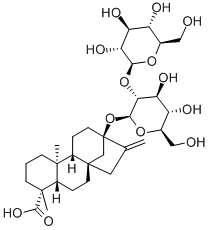
- Chemical Name:steviolbioside
- CAS:41093-60-1
- MF:C32H50O13
- Structure:
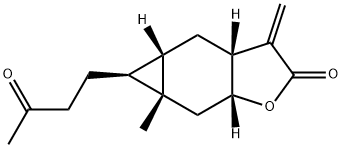
- Chemical Name:Carabrone
- CAS:1748-81-8
- MF:C15H20O3
- Structure:
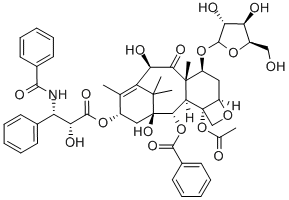
- Chemical Name:10-DEACETYL-7-XYLOSYLPACLITAXEL
- CAS:90332-65-3
- MF:C49H63NO17
- Structure:
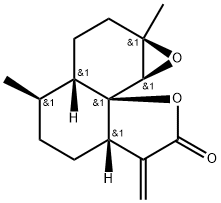
- Chemical Name:Arteannuin
- CAS:50906-56-4
- MF:C15H20O3
- Structure:
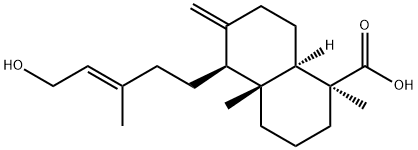
- Chemical Name:isocupressic acid
- CAS:1909-91-7
- MF:C20H32O3
- Structure:
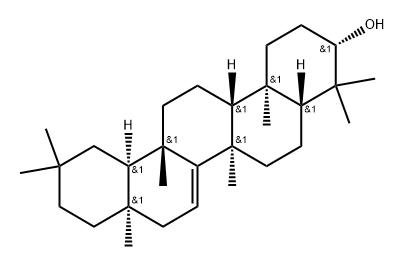
- Chemical Name:taraxerol
- CAS:127-22-0
- MF:C30H50O
- Structure:
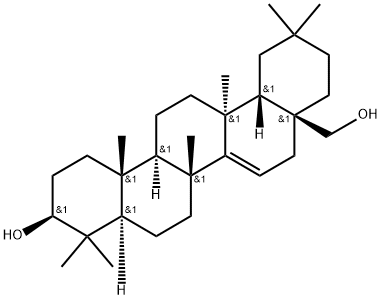
- Chemical Name:D-Friedoolean-14-ene-3β,28-diol
- CAS:17884-88-7
- MF:C30H50O2
- Structure:
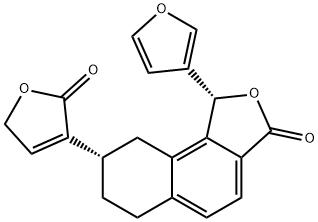
- Chemical Name:(1R,8S)-1α-(3-Furanyl)-6,7,8,9-tetrahydro-8α-[(2,5-dihydro-2-oxofuran)-3-yl]naphtho[1,2-c]furan-3(1H)-one
- CAS:126724-95-6
- MF:C20H16O5
- Structure:
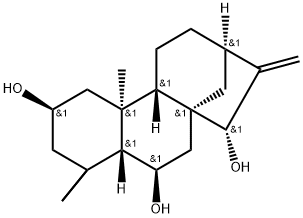
- Chemical Name:16-Kaurene-2,6,15-triol
- CAS:53452-32-7
- MF:C20H32O3
- Structure:
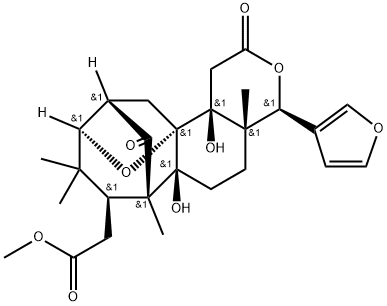
- Chemical Name:6-Deoxy-9alpha-hydroxycedrodorin
- CAS:247036-52-8
- MF:C27H34O9
- Structure:
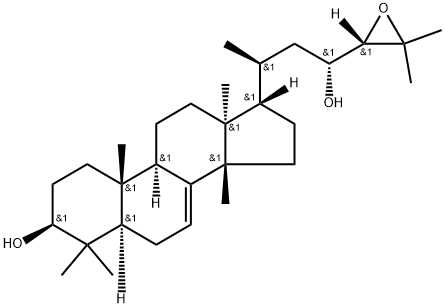
- Chemical Name:Dihydroniloticin
- CAS:115334-05-9
- MF:C30H50O3
- Structure:
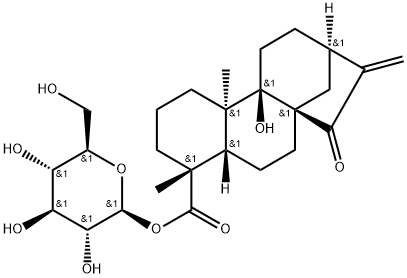
- Chemical Name:ent-9-Hydroxy-15-oxo-16-kauren- 19-oic acid beta-D-glucopyrasyl ester
- CAS:81263-96-9
- MF:C26H38O9
- Structure:
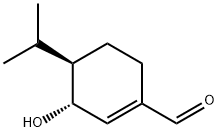
- Chemical Name:Eucamalol
- CAS:145544-91-8
- MF:C10H16O2
- Structure:
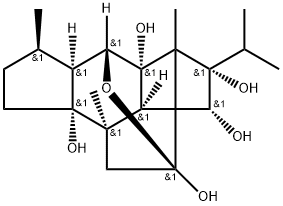
- Chemical Name:Itol A
- CAS:1033747-78-2
- MF:C20H32O6
- Structure:
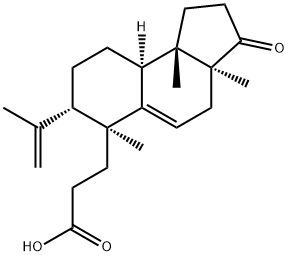
- Chemical Name:Micraic acid A
- CAS:659738-08-6
- MF:C22H32O3
- Structure:
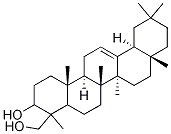
- Chemical Name:Olean-12-ene-3,24-diol
- CAS:119318-15-9
- MF:C30H50O2
- Structure:
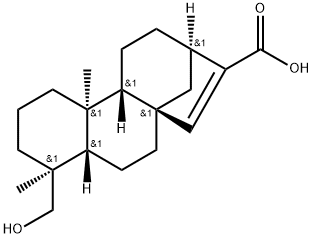
- Chemical Name:Pseudolaric acid D
- CAS:115028-67-6
- MF:C20H30O3
- Structure:
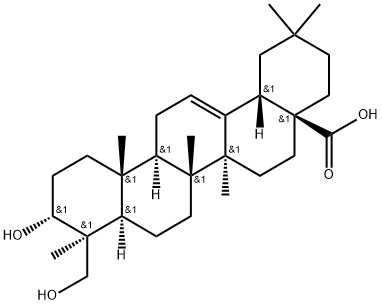
- Chemical Name:Scutellaric acid
- CAS:102919-76-6
- MF:C30H48O4
- Structure:
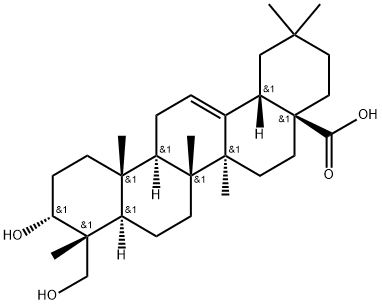
- Chemical Name:Wilforol C
- CAS:168254-95-3
- MF:C30H48O4
- Structure:
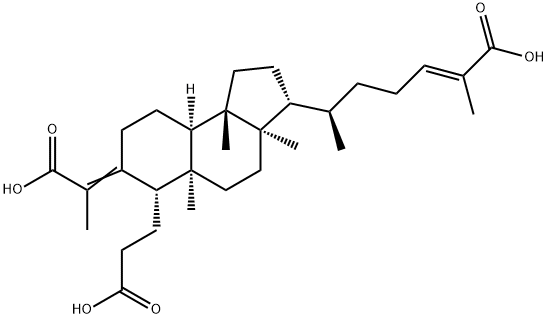
- Chemical Name:3,4-Secocucurbita-4,24-diene-3,26,29-trioic acid
- CAS:329975-47-5
- MF:C30H46O6
- Structure:
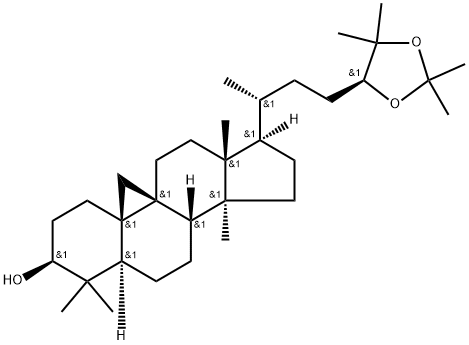
- Chemical Name:(24S)-Cycloartane-3,24,25-triol 24,25-acetonide
- CAS:57576-31-5
- MF:C33H56O3
- Structure:
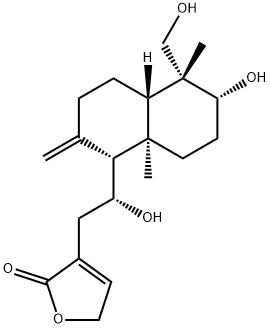
- Chemical Name:14-Deoxy-11-hydroxyandrographolide
- CAS:160242-09-1
- MF:C20H30O5
- Structure:
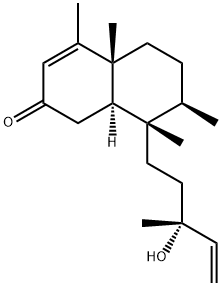
- Chemical Name:2-Oxokolavelool
- CAS:221466-41-7
- MF:C20H32O2
- Structure:
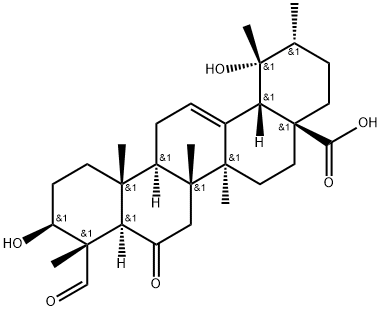
- Chemical Name:3,19-Dihydroxy-6,23-dioxo-12-ursen-28-oic acid
- CAS:261768-88-1
- MF:C30H44O6
- Structure:
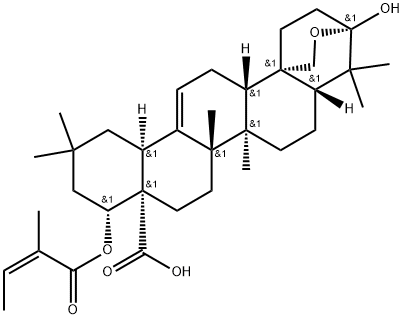
- Chemical Name:CaMaric acid
- CAS:146450-83-1
- MF:C35H52O6
- Structure:
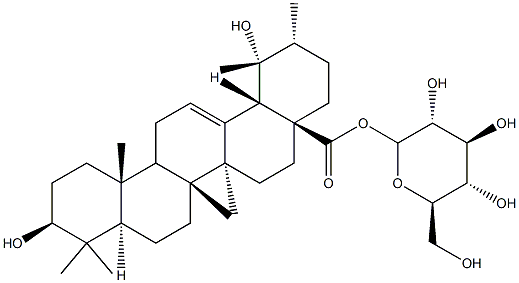
- Chemical Name:PoMolic acid 28-O-beta-D-glucopyranosyl ester
- CAS:83725-24-0
- MF:C36H58O9
- Structure:
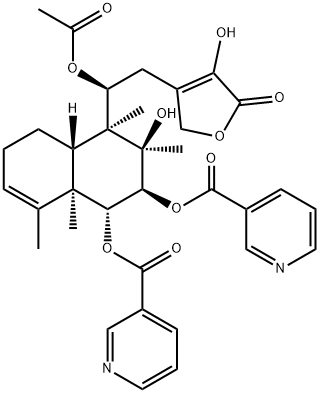
- Chemical Name:Scutebarbatine X
- CAS:1312716-26-9
- MF:C34H38N2O10
- Structure:
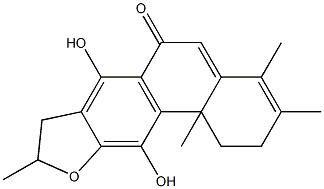
- Chemical Name:Uncinatone
- CAS:99624-92-7
- MF:C20H22O4
- Structure:
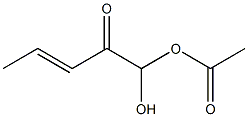
- Chemical Name:β-AMyrenonol acetate
- CAS:5356-56-9
- MF:C32H50O3
- Structure:
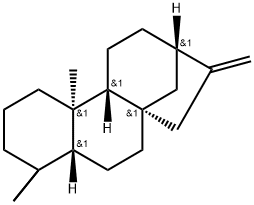
- Chemical Name:DACRENE
- CAS:20070-61-5
- MF:C20H32
- Structure:
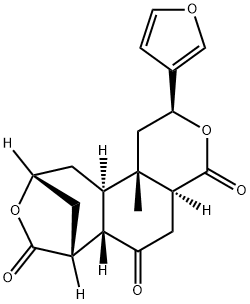
- Chemical Name:(2S)-2β-(3-Furyl)-4aα,5,6aβ,7,10,11,11aα,11b-octahydro-11bβ-methyl-7β,10β-methano-2H-pyrano[4,3-g][3]benzoxepine-4,6,8(1H)-trione
- CAS:66756-57-8
- MF:C19H20O6
- Structure:
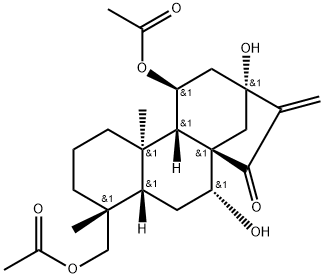
- Chemical Name:Rosthornin B
- CAS:125181-21-7
- MF:C24H34O7
- Structure:
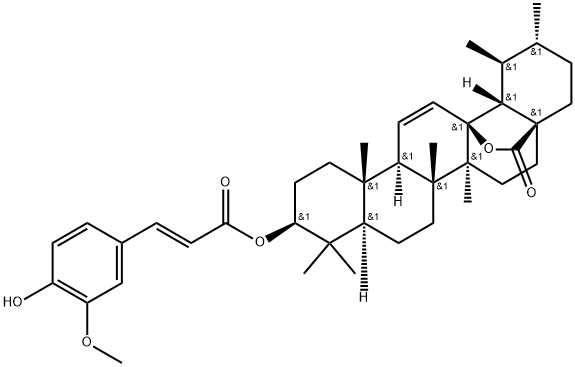
- Chemical Name:Tereticornate A
- CAS:149751-81-5
- MF:C40H54O6
- Structure:
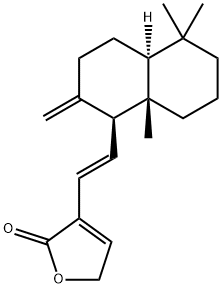
- Chemical Name:Villosin
- CAS:160598-92-5
- MF:C20H28O2
- Structure:
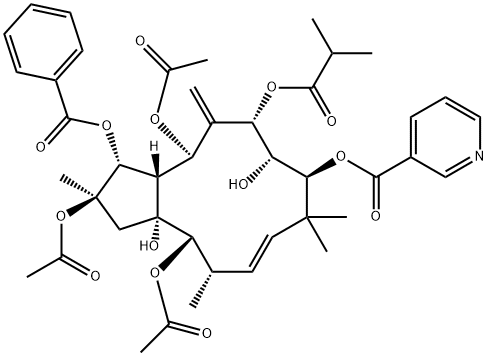
- Chemical Name:2,5,14-Triacetoxy-3-benzoyloxy-8,15-dihydroxy-7-isobutyroyloxy-9-nicotinoyloxyjatropha-6(17),11E-diene
- CAS:210108-87-5
- MF:C43H53NO14
- Structure:
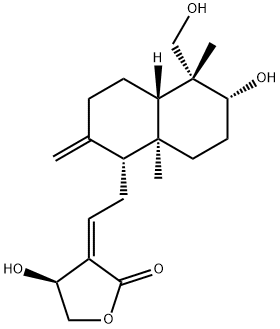
- Chemical Name:Andropanolide
- CAS:869807-57-8
- MF:C20H30O5
- Structure:
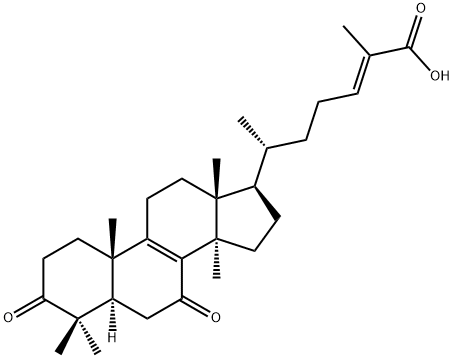
- Chemical Name:GANODERIC ACID DM
- CAS:173075-45-1
- MF:C30H44O4
- Structure:
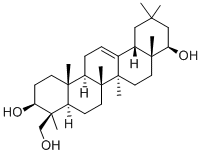
- Chemical Name:SOYASAPOGENOL B(P)
- CAS:595-15-3
- MF:C30H50O3
- Structure:
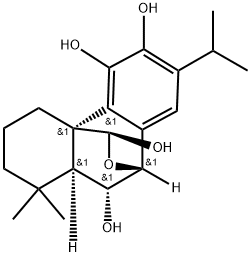
- Chemical Name:6-Epidemethylesquirolin D
- CAS:165074-00-0
- MF:C20H28O5
- Structure:
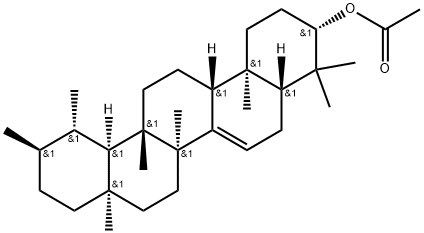
- Chemical Name:Bauerel acetate
- CAS:17020-04-1
- MF:C32H52O2
- Structure:
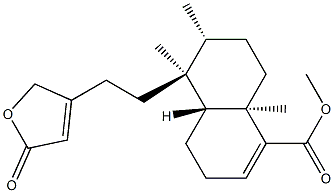
- Chemical Name:Clerodermic acid methyl ester
- CAS:67650-47-9
- MF:C21H30O4
- Structure:
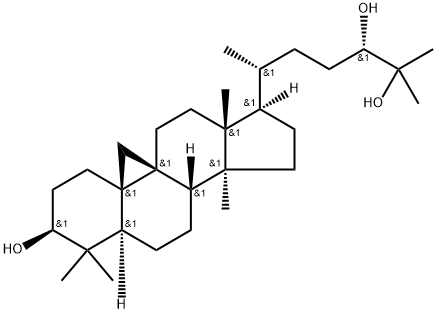
- Chemical Name:Cycloartane-3,24,25-triol
- CAS:57576-29-1
- MF:C30H52O3
- Structure:
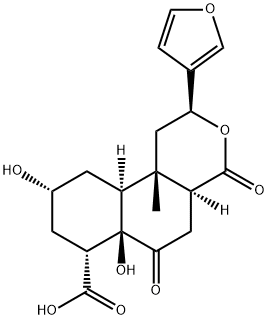
- Chemical Name:Diosbulbin J
- CAS:1187951-06-9
- MF:C19H22O8
- Structure:
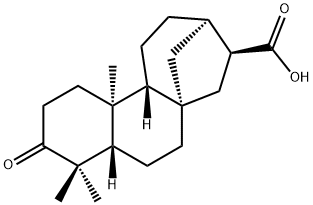
- Chemical Name:ent-3-Oxokauran-17-oic acid
- CAS:151561-88-5
- MF:C20H30O3
- Structure:
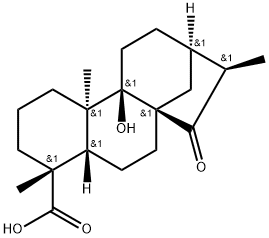
- Chemical Name:ent-9-Hydroxy-15-oxo-19-kauraic acid
- CAS:77658-45-8
- MF:C20H30O4
- Structure:
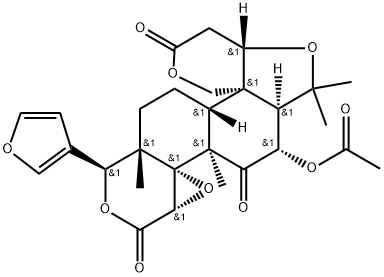
- Chemical Name:Glaucin B
- CAS:115458-73-6
- MF:C28H32O10
- Structure:
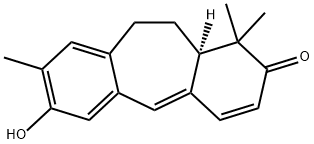
- Chemical Name:Heudelotine
- CAS:133453-58-4
- MF:C18H20O2
- Structure:
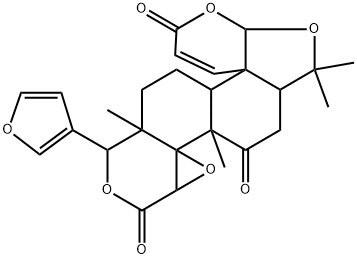
- Chemical Name:Jangomolide
- CAS:93767-25-0
- MF:C26H28O8
- Structure:
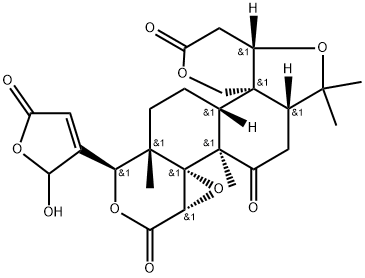
- Chemical Name:Limonexic acid
- CAS:99026-99-0
- MF:C26H30O10