Desulfurizer
Selective fluor-reagent-FTB is primarily taken as selective fluorinating agent and can have single-fluorination reaction with electron-rich double bond, enol ethers and enol lithium for the preparation of fluorine-containing steroid drugs.
Applications
1, direct fluorination
Direct fluorination refers to the direction introduction of fluorine to the non-fluoro substrate through electrophilic or nucleophilic fluorinating agent. Various forms of direct or indirect fluorinating reagent can react with certain groups of the substrates, thereby producing the desired fluorine-containing compound. General common fluorinating reagents comprising: (A) nucleophilic fluorinating reagent, such as HF, n-Bu4NF, SF4, DAST, etc., as well as metal fluorides NaF, KF, AgF, HgF2, SbF3 and so on. (B) Electrophilic or free radical electrophilic fluorinating reagents, including F2, XeF2, CFC acid (FClO3), CF3OF and "N? F" agent (including Selectfluor?, NFSI etc.), for example, in 2005, MacMillan group has reported of small organic molecule catalyzed asymmetric electrophilic fluorination reaction using NFSI as fluorination reagent (Scheme 2).
However, in the method of direct fluorination, these fluorinated reagents are either too toxic or expensive, and some of the reaction conditions are fierce and difficult to control with poor selectivity, causing limitation of introducing fluorine atom to some kinds of special molecules or the particular location of special molecules.
2, fluorine reagent colorimetric method
The fluoride in the food diffusion can have interaction with the acid in the box to produce hydrogen fluoride gas, further absorbed by the sodium hydroxide through diffusion. Fluoride can react with lanthanum, fluoride agent (Alizarin) to generate blue ternary complex at a suitable pH with the color being deepened with increasing fluoride ion concentration. Apply amine- or non amine-containing organic solvent for extraction and do quantitative comparison of standard series. Method applying amine-containing organic extraction is monochromatic method. This method has a relative high sensitivity with the limit of detection being 0.1 mg/kg; using non-amine-containing organic reagent for extraction belongs to multi-color method with simple operation and the minimum detectable amount being 0.2mg / kg.
3, Fluor reagent spectrophotometry
One kind of method for determining the fluoride content is surface water, groundwater and industrial effluent. The basic principle is: the fluoride ion can form blue ternary complex with fluorine and lanthanum nitrate reagents in the HCl buffered medium (pH4.1) with the color intensity being proportional to the fluoride concentration. Apply this for quantitative determination of fluoride concentration at a wavelength of 620nm content (in F- unit). In the 25 mg chromogenic solution containing 5g of fluoride, upon the presence of the following ions exceeding the following content (in mg), there will be interference on the determination, but can be eliminated by pre-distillation: Cl-30; SO42-5.0; NO3-3.0; B4O72 -2.0; Mg2 + 2.0; NH4 + 1.0; Ca2 + 0.5. The minimum detectable concentration of fluoride containing compound is 0.05mg /L (in F- unit) but the upper limit of the measured concentration is 1.80 mg/L. This method has been set as a national standard analytical method (GB7483-87).
Fluor reagent [classification]
(1) Deoxo fluorination
Deoxo-Fluor is a versatile, easy to use and more secure nucleophilic fluorination reagent. It can quickly "deoxy-fluoridize" various kinds of alcohols, aldehydes and ketones under mild conditions to synthesize corresponding fluoride with high efficiency and high selectivity. The thermal sensitivity of the Deoxo-Fluor reagent is lower than that of conventional Deoxo-Fluor reagent, therefore being more suitable for large-scale applications.
(2) Electrophilic fluorination
Select fluor series reagent is a series of N-F type electrophilic fluoride and can be used in pharmaceutical and agricultural applications. The series includes Selectfluor I and Selectfluor II reagents. Both reagents are versatile and easy to use security agents, capable of fluorinate the specific locations of complex molecules. Selectfluor reagent can selectively, rapidly and efficiently fluorinate various kinds of organic molecules under mild conditions. In many cases, Select fluor reagent can substitute FClO3 or CF3OF and other kinds of toxic or explosive fluorinated reagents. Select fluor reagents are convenient and simple substitute available for your fluorination technology process.
Medicine and agriculture industry has tested and applied various kinds of fluorinating reagent in limited circumstances, including the N-F type electron affinity reagent. However, there are many reagents having severe limitations. Some agents have been proven costly and difficult to manufacture, and therefore does not have economic viability. Attempted other fluorinated reagents, due to lack of efficient fluoride, make it difficult to synthesize cost-effective chemicals or demand addition processing steps for removing the impurities arising from the process.
Select fluor reagent is able to solve all these problems. Selectfluor reagents have adopted convenient-amount packaging, being easy for evaluation and commercial production.
Source of this type of information: Air Products Product is a world leading industrial gases company.
- Structure:
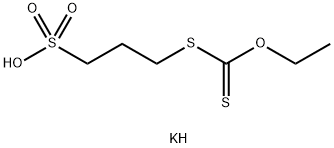
- Chemical Name:(O-ETHYLDITHIOCARBONATO)-S-(3-SULFOPROPYL)-ESTER, POTASSIUM SALT
- CAS:93841-14-6
- MF:C6H13KO4S3
- Chemical Name:Desulfurizer
- CAS:
- MF:2ZnCO3-3ZN(OH)2-2H2O
- Chemical Name:Arsenic-remover
- CAS:
- MF: