Silane reagent
Silane reagent refers to a class of organic silicon monomer or small molecule compounds in organic synthesis or analysis or for the protection of organic change in the active group. After the reaction of the silane groups falling restitution. There are more than 500 species, including:
(1) The trimethylsilyl group the SBA monofunctional, e.g. trimethylchlorosilane, bis (trimethylsilyl) amine and the like.
(2) the SBA monoenergetic sterically hindered, such as tert-butyldimethylsilyl chloride, triisopropyl chloro silane.
(3) the SBA difunctional sterically hindered, such as dimethyl acetoxy silane, di-t-butyl dichloro silane.
(4) Other protective agent, such as trimethyl silane-hydroxyethyl, hydroxyethyl methyl diphenyl silane.
Silane reagent can be used to analyze the field of organic synthesis (eg pharmaceutical synthesis). Modified drugs may also be used, such as to make it oil-soluble or loss of bitterness and the like.
Refers to the effect of silylation silyl group introduced into the molecule, usually an active hydrogen-substituted (eg: hydroxyl -OH, carboxyl groups -COOH, -NH2 in an amino group, a mercapto group -SH, phosphate). After the active hydrogen group substituted with a silane reducing polar compounds, to reduce the hydrogen bondage, silylated derivative thus formed is more volatile; Meanwhile, due to the reduced number of reactive sites of the active hydrogen-containing, but also stability of the compound can be strengthened. Silane compounds polar weakened, the measured capacity enhancement, improved thermal stability. Silylating agent in GC analysis uses a great many considered to be non-volatile or thermally unstable at 200-300 ℃ hydroxy or amino compounds successfully conducted chromatography after silylation.
It is precisely because the silane reagent to active hydrogen sensitive, reactive therewith happen, so the same silylating agent is very sensitive to moisture, water in the environment itself decomposition failure. Usually mentioned silylating agent, are sealed in nitrogen. Nitrogen must be pre-dried to remove water. After access to the silylating agent, again before the seal must charge into nitrogen. So that you can get rid of the air, to avoid the seal into the air so that the period of contact with moisture in the air with the silylating agent. Nitrogen filling method Proceed as follows: Connect a nitrogen cylinder with a Teflon tube at one end, connected at one end Pasteur pipette. The straw into his head to be filled with nitrogen silane reagent bottle from the reagent liquid silane of about 2 ~ 3cm. Open the nitrogen switch, so that nitrogen slowly into the vial until it has been filled with nitrogen, silane reagent bottle of white space. Removed the straw and immediately sealed tight bottle, close nitrogen.
Trimethylsilyl imidazole silane is strongest hydroxy silylating agent; quickly, smoothly and carboxyl groups react with hydroxyl groups. Does not react with amines or amides, it can be used to prepare both a hydroxy and an amino derivative containing multiple compounds. In the presence of a small amount of water it can be used silane sugar; when you need to analyze the sugar syrup as the sugar is ideal for silylation. Derivatives can not be hindered and the most serious impediment to steroid hydroxyl groups.
- Structure:
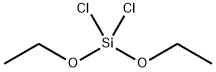
- Chemical Name:DIETHOXYDICHLOROSILANE
- CAS:4667-38-3
- MF:C4H10Cl2O2Si
- Structure:
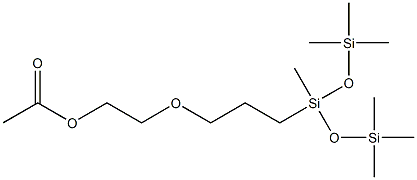
- Chemical Name:2-[ACETOXY(POLYETHYLENEOXY)PROPYL]HEPTAMETHYLTRISILOXANE
- CAS:125997-17-3
- MF:C14H34O5Si3
- Structure:
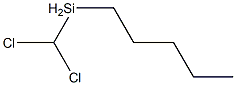
- Chemical Name:N-AMYLMETHYLDICHLOROSILANE
- CAS:13682-99-0
- MF:C6H14Cl2Si
- Structure:
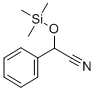
- Chemical Name:ALPHA-(TRIMETHYLSILYLOXY)PHENYLACETONIT&
- CAS:25438-37-3
- MF:C11H15NOSi
- Structure:
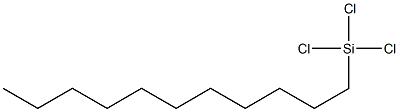
- Chemical Name:N-UNDECYLTRICHLOROSILANE
- CAS:18052-07-8
- MF:C11H23Cl3Si
- Structure:
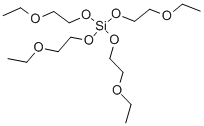
- Chemical Name:TETRAKIS(ETHOXYETHOXY)SILANE
- CAS:18407-94-8
- MF:C16H36O8Si
- Structure:
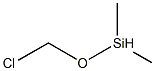
- Chemical Name:DIMETHYLMETHOXYCHLOROSILANE
- CAS:1825-68-9
- MF:C3H9ClOSi
- Structure:
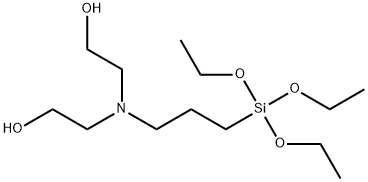
- Chemical Name:BIS(2-HYDROXYETHYL)-3-AMINOPROPYLTRIETHOXYSILANE
- CAS:7538-44-5
- MF:C13H31NO5Si
- Structure:
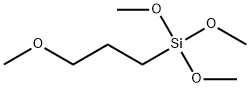
- Chemical Name:3-(METHOXY)PROPYLTRIMETHOXYSILANE
- CAS:33580-59-5
- MF:C7H18O4Si
- Structure:
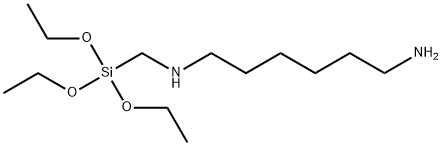
- Chemical Name:N-(6-AMINOHEXYL)AMINOMETHYLTRIETHOXYSILANE
- CAS:15129-36-9
- MF:C13H32N2O3Si
- Structure:
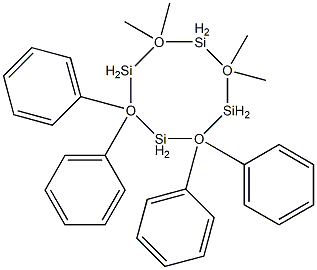
- Chemical Name:1,1,3,3-Tetramethyltetraphenylcyclotetrasiloxane
- CAS:1693-47-6
- MF:C28H32O4Si4
- Structure:
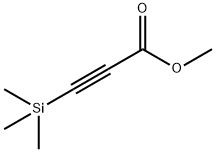
- Chemical Name:METHYL 3-(TRIMETHYLSILYL)PROPIOLATE
- CAS:42201-71-8
- MF:C7H12O2Si
- Structure:
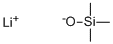
- Chemical Name:LITHIUM TRIMETHYLSILANOLATE
- CAS:2004-14-0
- MF:C3H9LiOSi
- Structure:
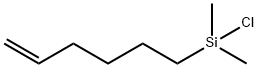
- Chemical Name:(5-HEXENYL)DIMETHYLCHLOROSILANE
- CAS:30102-73-9
- MF:C8H17ClSi
- Structure:
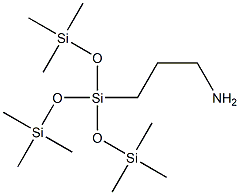
- Chemical Name:3-AMINOPROPYLTRIS(TRIMETHYLSILOXY)SILANE
- CAS:25357-81-7
- MF:C12H35NO3Si4
- Structure:
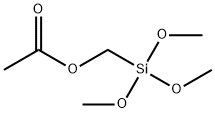
- Chemical Name:ACETOXYMETHYLTRIMETHOXYSILANE
- CAS:65625-39-0
- MF:C6H14O5Si
- Structure:
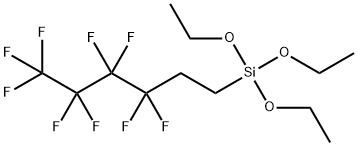
- Chemical Name:Nonafluorohexyltriethoxysilane
- CAS:102390-98-7
- MF:C12H19F9O3Si
- Structure:
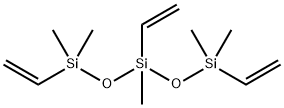
- Chemical Name:1,3,5-TRIVINYL-1,1,3,5,5-PENTAMETHYLTRISILOXANE
- CAS:1529-65-3
- MF:C11H24O2Si3
- Structure:
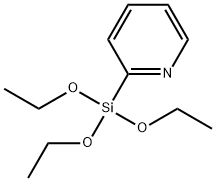
- Chemical Name:Pyridine, 2-(triethoxysilyl)- (9CI)
- CAS:213602-91-6
- MF:C11H19NO3Si
- Structure:
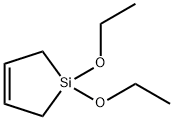
- Chemical Name:1,1-DIETHOXY-1-SILACYCLOPENT-3-ENE
- CAS:67059-49-8
- MF:C8H16O2Si
- Structure:
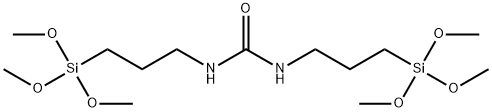
- Chemical Name:N,N-bis(3-Trimethoxysilylpropyl)urea
- CAS:18418-53-6
- MF:C13H32N2O7Si2
- Structure:
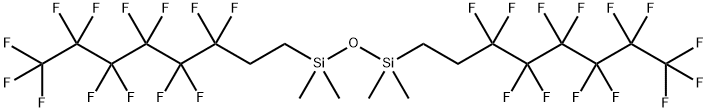
- Chemical Name:BIS(TRIDECAFLUORO-1,1,2,2-TETRAHYDROOCTYL)TETRAMETHYLDISILOXANE
- CAS:71363-70-7
- MF:C20H20F26OSi2
- Structure:
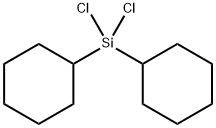
- Chemical Name:DICYCLOHEXYLDICHLOROSILANE
- CAS:18035-74-0
- MF:C12H22Cl2Si
- Structure:
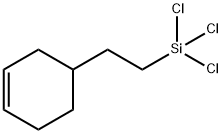
- Chemical Name:[2-(3-CYCLOHEXENYL)ETHYL]TRICHLOROSILANE
- CAS:18290-60-3
- MF:C8H13Cl3Si
- Structure:
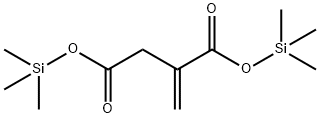
- Chemical Name:BIS(TRIMETHYLSILYL)ITACONATE
- CAS:55494-04-7
- MF:C11H22O4Si2
- Structure:
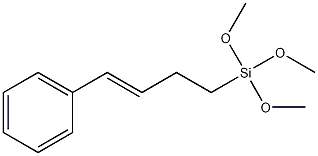
- Chemical Name:STYRYLETHYLTRIMETHOXYSILANE ,tech-90
- CAS:119181-19-0
- MF:C13H20O3Si
- Structure:
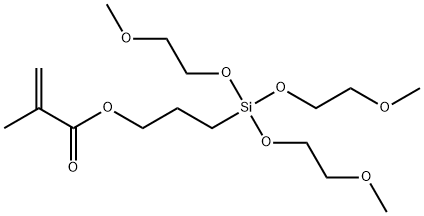
- Chemical Name:3-METHACRYLOXYPROPYLTRIS(METHOXYETHOXY)SILANE
- CAS:57069-48-4
- MF:C16H32O8Si
- Structure:
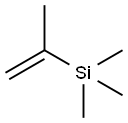
- Chemical Name:2-PROPENYLTRIMETHYLSILANE
- CAS:18163-07-0
- MF:C6H14Si
- Structure:
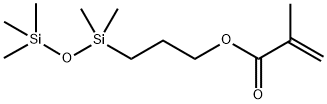
- Chemical Name:3-METHACRYLOXYPROPYLPENTAMETHYLDISILOXANE
- CAS:18151-85-4
- MF:C12H26O3Si2
- Structure:
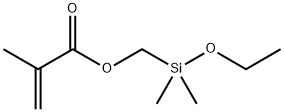
- Chemical Name:(METHACRYLOXYMETHYL)DIMETHYLETHOXYSILANE
- CAS:5577-70-8
- MF:C9H18O3Si
- Structure:
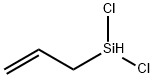
- Chemical Name:ALLYLDICHLOROSILANE
- CAS:3937-28-8
- MF:C3H6Cl2Si
- Structure:
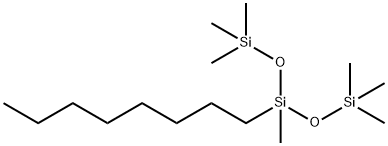
- Chemical Name:3-OCTYLHEPTAMETHYLTRISILOXANE
- CAS:17955-88-3
- MF:C15H38O2Si3
- Structure:
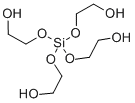
- Chemical Name:TETRAGLYCOLATOSILANE
- CAS:17622-94-5
- MF:C8H20O8Si
- Structure:
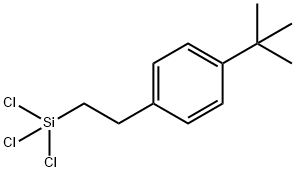
- Chemical Name:P-(T-BUTYL)PHENETHYLTRICHLOROSILANE
- CAS:211925-40-5
- MF:C12H17Cl3Si
- Structure:
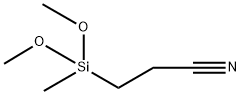
- Chemical Name:2-Cyanoethylmethyldimethoxysilane
- CAS:2526-61-6
- MF:C6H13NO2Si
- Structure:
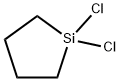
- Chemical Name:CYCLOTETRAMETHYLENEDICHLOROSILANE
- CAS:2406-33-9
- MF:C4H8Cl2Si
- Structure:
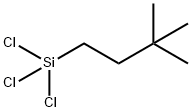
- Chemical Name:(3,3-DIMETHYLBUTYL)TRICHLOROSILANE
- CAS:105732-02-3
- MF:C6H13Cl3Si
- Structure:
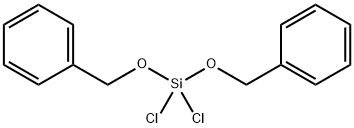
- Chemical Name:DIBENZYLOXYDICHLOROSILANE
- CAS:18414-52-3
- MF:C14H14Cl2O2Si
- Structure:
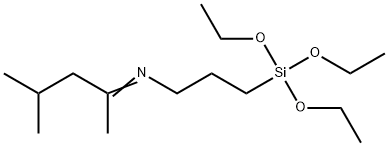
- Chemical Name:3-(1,3-DIMETHYLBUTYLIDENE)AMINOPROPYLTRIETHOXYSILANE
- CAS:116229-43-7
- MF:C15H33NO3Si
- Structure:
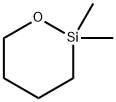
- Chemical Name:1,1-DIMETHYL-1-SILA-2-OXACYCLOHEXANE
- CAS:5833-47-6
- MF:C6H14OSi
- Structure:

- Chemical Name:PRASEODYMIUM ZIRCONIUM SILICATE
- CAS:68187-15-5
- MF:O21Pr2Si7Zr2
- Structure:
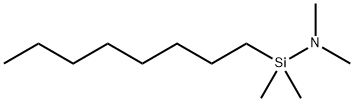
- Chemical Name:N-OCTYLDIMETHYL (DIMETHYLAMINO) SILANE
- CAS:110348-62-4
- MF:C12H29NSi
- Structure:
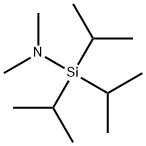
- Chemical Name:(DIMETHYLAMINO)TRIISOPROPYLSILANE 96
- CAS:181231-66-3
- MF:C11H27NSi
- Structure:
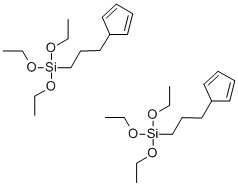
- Chemical Name:(3-CYCLOPENTADIENYLPROPYL)TRIETHOXYSILANE - DIMER
- CAS:102056-64-4
- MF:C28H52O6Si2
- Structure:
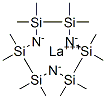
- Chemical Name:LANTHANUM TRIS[BIS(TRIMETHYLSILYL)AMIDE]
- CAS:35788-99-9
- MF:C18H54LaN3Si6
- Structure:
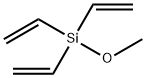
- Chemical Name:TRIVINYLMETHOXYSILANE
- CAS:193828-96-5
- MF:C7H12OSi
- Structure:
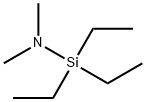
- Chemical Name:(N,N-DIMETHYLAMINO)TRIETHYLSILANE
- CAS:3550-35-4
- MF:C8H21NSi
- Structure:
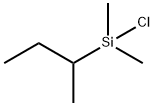
- Chemical Name:CHLORODIMETHYLISOBUTYLSILANE
- CAS:60090-96-2
- MF:C6H15ClSi
- Structure:
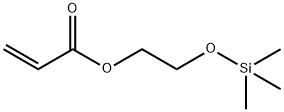
- Chemical Name:2-(ACRYLOXYETHOXY)TRIMETHYLSILANE
- CAS:18269-99-3
- MF:C8H16O3Si
- Structure:
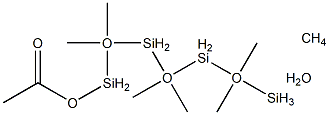
- Chemical Name:ACETOXYHEPTAMETHYLCYCLOTETRASILOXANE
- CAS:14697-86-0
- MF:C9H24O6Si4
- Structure:
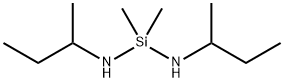
- Chemical Name:DIMETHYLBIS(S-BUTYLAMINO)SILANE
- CAS:93777-98-1
- MF:C10H26N2Si
- Structure:
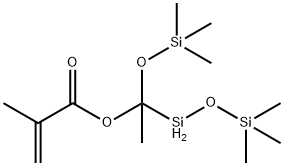
- Chemical Name:(METHACRYLOXYMETHYL)BIS(TRIMETHYLSILOXY)METHYLSILANE
- CAS:18166-40-0
- MF:C12H28O4Si3
- Structure:
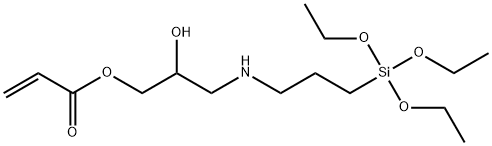
- Chemical Name:N-(3-ACRYLOXY-2-HYDROXYPROPYL)-3-AMINOPROPYLTRIETHOXYSILANE
- CAS:123198-57-2
- MF:C15H31NO6Si
- Structure:
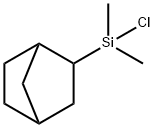
- Chemical Name:2-(BICYCLOHEPTYL)DIMETHYLCHLOROSILANE
- CAS:117046-42-1
- MF:C9H17ClSi
- Structure:
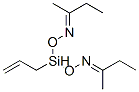
- Chemical Name:VINYLMETHYLBIS(METHYLETHYLKETOXIMINO)SILANE
- CAS:73160-32-4
- MF:C11H22N2O2Si
- Structure:
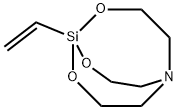
- Chemical Name:1-VINYLSILATRANE
- CAS:2097-18-9
- MF:C8H15NO3Si
- Structure:
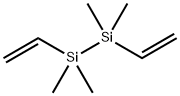
- Chemical Name:DIVINYLTETRAMETHYLDISILANE
- CAS:1450-29-9
- MF:C8H18Si2
- Structure:
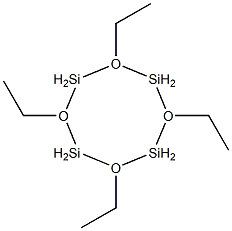
- Chemical Name:TETRAETHYLCYCLOTETRASILOXANE
- CAS:16066-10-7
- MF:C8H24O4Si4
- Structure:
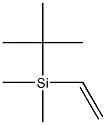
- Chemical Name:VINYL-T-BUTYLDIMETHYLSILANE
- CAS:24858-02-4
- MF:C8H18Si
- Structure:
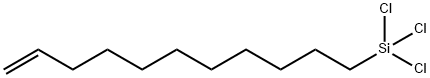
- Chemical Name:10-UNDECENYLTRICHLOROSILANE
- CAS:17963-29-0
- MF:C11H21Cl3Si
- Structure:
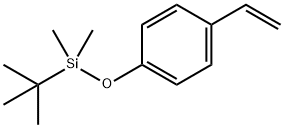
- Chemical Name:P-(T-BUTYLDIMETHYLSILOXY)STYRENE
- CAS:84494-81-5
- MF:C14H22OSi
- Structure:
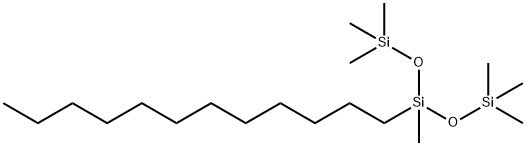
- Chemical Name:LAURYL METHICONE
- CAS:139614-44-1
- MF:C19H46O2Si3
- Structure:
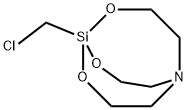
- Chemical Name:CHLOROMETHYLSILATRANE
- CAS:42003-39-4
- MF:C7H14ClNO3Si
- Structure:
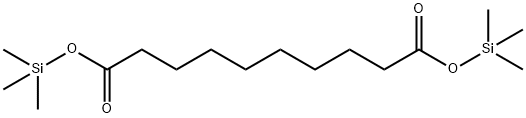
- Chemical Name:BIS(TRIMETHYLSILYL)SEBACATE
- CAS:18408-42-9
- MF:C16H34O4Si2
- Structure:
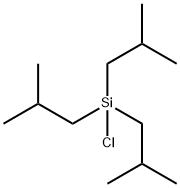
- Chemical Name:TRIISOBUTYLCHLOROSILANE
- CAS:13154-25-1
- MF:C12H27ClSi
- Structure:
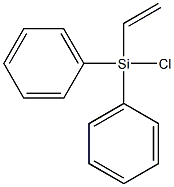
- Chemical Name:DIPHENYLVINYLCHLOROSILANE
- CAS:18419-53-9
- MF:C14H13ClSi
- Structure:
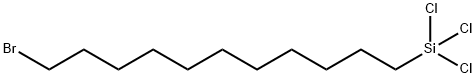
- Chemical Name:11-BROMOUNDECYLTRICHLOROSILANE
- CAS:79769-48-5
- MF:C11H22BrCl3Si
- Structure:
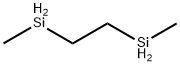
- Chemical Name:1,2-ETHANEDIYLBIS(METHYLSILANE)
- CAS:4405-22-5
- MF:C4H14Si2
- Structure:
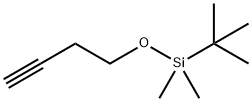
- Chemical Name:4-(T-BUTYLDIMETHYLSILOXY)BUTYNE
- CAS:78592-82-2
- MF:C10H20OSi
- Structure:
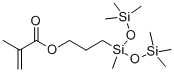
- Chemical Name:METHACRYLOXYPROPYLBIS(TRIMETHYLSILOXY)METHYLSILANE
- CAS:19309-90-1
- MF:C14H32O4Si3
- Structure:
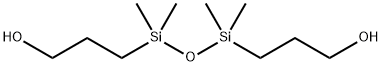
- Chemical Name:1,3-BIS(3-HYDROXYPROPYL)TETRAMETHYLDISILOXANE
- CAS:18001-97-3
- MF:C10H26O3Si2
- Structure:
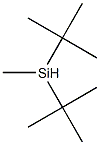
- Chemical Name:DI-T-BUTYLMETHYLSILANE
- CAS:56310-20-4
- MF:C9H22Si
- Structure:
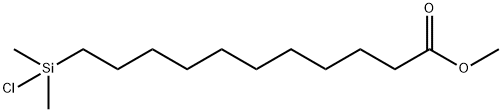
- Chemical Name:(10-CARBOMETHOXYDECYL)DIMETHYLCHLOROSILANE
- CAS:53749-38-5
- MF:C14H29ClO2Si
- Structure:
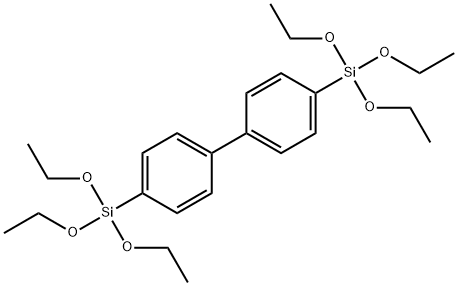
- Chemical Name:4 4'-BIS(TRIETHOXYSILYL)-1 1'-BIPHENYL
- CAS:123640-93-7
- MF:C24H38O6Si2
- Structure:

- Chemical Name:trimethoxy-[3-(2-methoxyethoxy)propyl]silane
- CAS:65994-07-2
- MF:C9H22O5Si
- Structure:
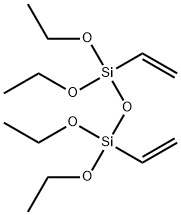
- Chemical Name:1,3-DIVINYLTETRAETHOXYDISILOXANE
- CAS:3682-26-6
- MF:C12H26O5Si2
- Structure:
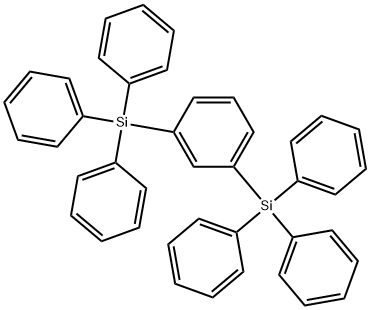
- Chemical Name:1,3-Bis(triphenylsilyl)benzene
- CAS:18920-16-6
- MF:C42H34Si2
- Structure:
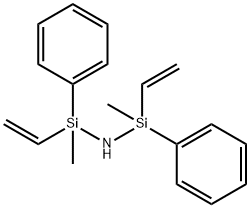
- Chemical Name:1,3-DIVINYL-1,3-DIPHENYL-1,3-DIMETHYLDISILAZANE
- CAS:23038-10-0
- MF:C18H23NSi2
- Structure:
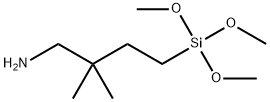
- Chemical Name:4-AMINO-3,3-DIMETHYLBUTYLTRIMETHOXYSILANE
- CAS:157923-74-5
- MF:C9H23NO3Si
- Structure:
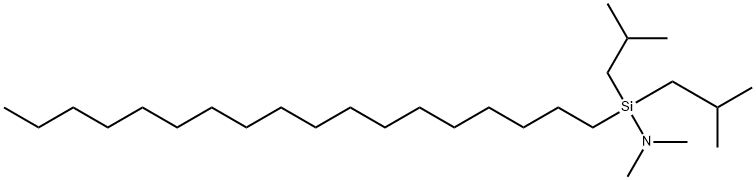
- Chemical Name:OCTADECYL DIISOBUTYL (DIMETHYL AMINO) SILANE
- CAS:151613-23-9
- MF:C28H61NSi
- Structure:
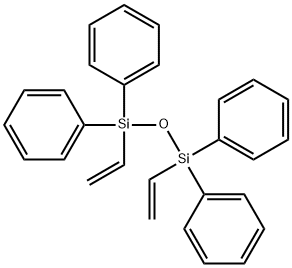
- Chemical Name:DIVINYLTETRAPHENYLDISILOXANE
- CAS:18769-05-6
- MF:C28H26OSi2
- Structure:
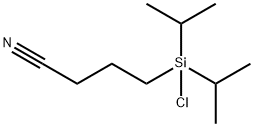
- Chemical Name:3-CYANOPROPYLDIISOPROPYLCHLOROSILANE
- CAS:113641-37-5
- MF:C10H20ClNSi
- Structure:
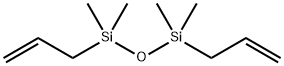
- Chemical Name:1,3-DIALLYLTETRAMETHYLDISILOXANE
- CAS:17955-81-6
- MF:C10H22OSi2
- Structure:
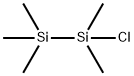
- Chemical Name:CHLOROPENTAMETHYLDISILANE
- CAS:1560-28-7
- MF:C5H15ClSi2
- Structure:
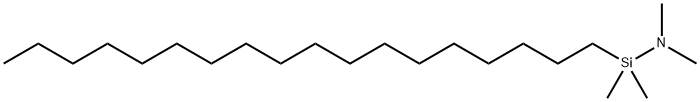
- Chemical Name:N-OCTADECYLDIMETHYL(DIMETHYLAMINO)SILANE
- CAS:76328-77-3
- MF:C22H49NSi
- Structure:
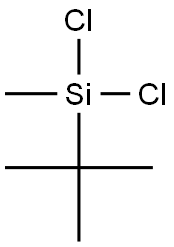
- Chemical Name:DICHLOROISOBUTYLMETHYLSILANE
- CAS:18147-18-7
- MF:C5H12Cl2Si
- Structure:
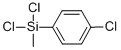
- Chemical Name:CHLOROPHENYLMETHYLDICHLOROSILANE
- CAS:25898-35-5
- MF:C7H7Cl3Si
- Structure:
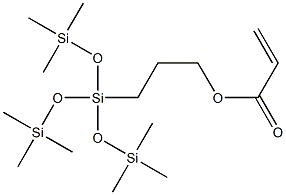
- Chemical Name:(3-ACRYLOXYPROPYL)TRIS(TRIMETHYLSILOXY)-SILANE
- CAS:17096-12-7
- MF:C15H36O5Si4
- Structure:
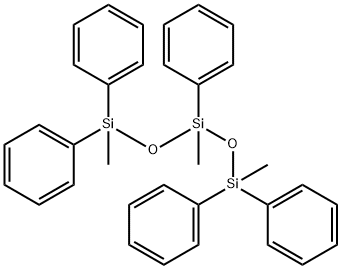
- Chemical Name:1,1,3,5,5-Pentaphenyl-1,3,5-trimethyltrisiloxane
- CAS:3390-61-2
- MF:C33H34O2Si3
- Structure:
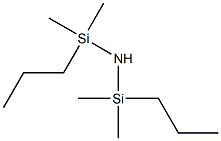
- Chemical Name:1,3-DI-N-PROPYL-1,1,3,3-TETRAMETHYLDISILAZANE
- CAS:14579-90-9
- MF:C10H27NSi2
- Structure:
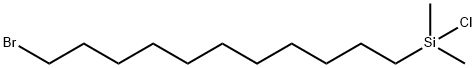
- Chemical Name:11-BROMOUNDECYLDIMETHYLCHLOROSILANE
- CAS:330457-42-6
- MF:C13H28BrClSi
- Structure:
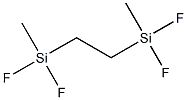
- Chemical Name:1,2-BIS(METHYLDIFLUOROSILYL)ETHANE
- CAS:170381-99-4
- MF:C4H10F4Si2
- Structure:
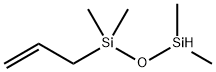
- Chemical Name:1-ALLYL-1,1,3,3-TETRAMETHYLDISILOXANE
- CAS:18387-26-3
- MF:C7H18OSi2
- Structure:

- Chemical Name:VINYLPHENYLDICHLOROSILANE
- CAS:7719-02-0
- MF:C8H8Cl2Si
- Structure:

- Chemical Name:N-BUTYLDIMETHYLMETHOXYSILANE
- CAS:64712-50-1
- MF:C7H18OSi
- Structure:
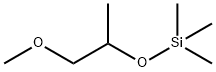
- Chemical Name:(1-METHOXY-2-PROPOXY)TRIMETHYLSILANE
- CAS:55816-62-1
- MF:C7H18O2Si
- Structure:
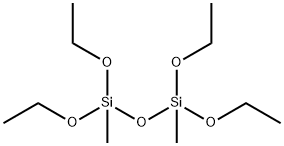
- Chemical Name:1,1,3,3-TETRAETHOXY-1,3-DIMETHYLDISILOXANE
- CAS:18001-60-0
- MF:C10H26O5Si2
- Structure:
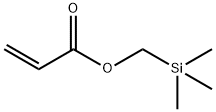
- Chemical Name:ACRYLOXYMETHYLTRIMETHYLSILANE
- CAS:67186-35-0
- MF:C7H14O2Si
- Structure:
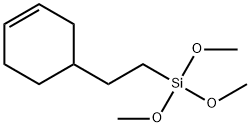
- Chemical Name:2-(3-CYCLOHEXENYL)ETHYLTRIMETHOXYSILANE
- CAS:67592-36-3
- MF:C11H22O3Si
- Structure:
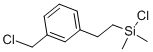
- Chemical Name:((CHLOROMETHYL)PHENYLETHYL)DIMETHYLCHLOROSILANE
- CAS:68092-71-7
- MF:C11H16Cl2Si