Oligosaccharides
Oligosaccharide is known as oligose, straight-chain or branched chain saccharide containing 2 to 10 identical or different monosaccharide units connected by glycosidic bond. The physical and chemical properties of oligosaccharide and monosaccharide are similar, they both are soluble in water, and many oligosaccharides have a sweet taste and can be oxidized by Fehling solution. The only difference between the two is that the oligosaccharides produce less than ten monosaccharide molecules after hydrolysis. According to the number of monosaccharide units oligosaccharides are divided into disaccharide, trisaccharide, tetrasaccharide and pentasaccharide and the like. The monosaccharides consisting oligosaccharides can be same or different, and the difference from the general glycoside is that the ligand of the oligosaccharide is sugar. Such as sucrose is a disaccharide, raffinose is a trisaccharide, stachyose is a four sugar, and mullein sugar is a five sugar. They are soluble in water or other polar solvents, with the discrimination of reductive and non-reductive. In vivo, oligosaccharide can be synthesized from monosaccharide gradually, or produced from polysaccharide degradation. Structure polysaccharides existing in plant cell wall can be degraded into biologically active fragments during the invasion of pathogens, so that the structure polysaccharides known as oligosaccharin can protect and regulate plants.
The most common trisaccharide is raffinose existing in beet and cottonseed. The syrup obtained from the process of manufacturing sucrose from beet is the best raw materials to extract raffinose. One molecule of galactose, glucose and fructose can be obtained after the hydrolysis of raffinose. Raffinose is a non-reduction sugar, glucose and fructose combine in hemiacetal hydroxyl way, while between the glucose and galactose is a 1,6 glycosidic bond, and the sequence of the combination can be expressed as follows: galactose - (1-> 6) - glucose - (1-> 2) - fructose.
Stachyose, a more important four sugar in the nature, is formed from adding a galactose to 6 → 1 glycosidic bond existing in raffinose molecule .In the same way, the α, D-galactose can be connected to glycosidic bond to form pentasaccharide, hexasaccharide and seven sugar homologues. Stachyose and its homologues are antifreeze substances for plants.
- Structure:
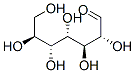
- Chemical Name:D-MANNOHEPTOSE
- CAS:7634-39-1
- MF:C7H14O7
- Structure:
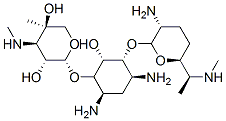
- Chemical Name:(2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-[(1R)-1-methylaminoethyl]oxan-2-yl]oxy-2-hydroxy-cyclohexyl]oxy-5-methyl-4-methylamino-oxane-3,5-diol
- CAS:25876-10-2
- MF:C21H43N5O7
- Structure:
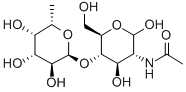
- Chemical Name:2-ACETAMIDO-2-DEOXY-4-O-(A-L-FUCOPYRANOSYL)-D-GLUCOPYRANOSE
- CAS:76211-71-7
- MF:C14H25NO10
- Structure:
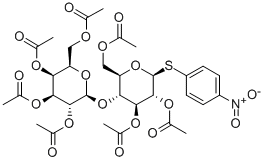
- Chemical Name:4-Nitrophenyl4-O-(2,3,4,6-tetra-O-acetyl-b-D-galactopyranosyl)-2,3,6-tri-O-acetyl-b-D-thioglucopyranoside
- CAS:27894-81-1
- MF:C32H39NO19S
- Structure:
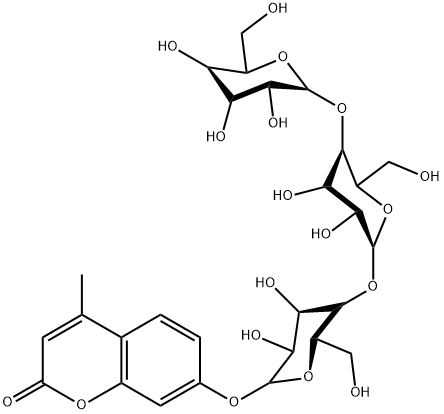
- Chemical Name:4-methylumbelliferyl-beta-D-cellotrioside
- CAS:84325-18-8
- MF:C28H38O18
- Structure:
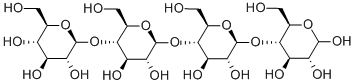
- Chemical Name:D-(+)-CELLOTETRAOSE
- CAS:38819-01-1
- MF:C24H42O21
- Structure:
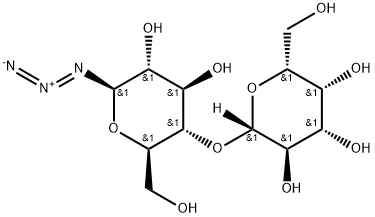
- Chemical Name:BETA-LACTOSYL AZIDE
- CAS:69266-16-6
- MF:C12H21N3O10