Natural amino acids and derivatives
The amino acids are basic composites of protein, being the carboxylates containing amino groups in the molecules. There are over 200 kinds of naturally existing amino acids. But the number of amino acids constituting the animal's body and its protein product is only 20.
Structure and classification of amino acids
The structure of amino acid: amino acids obtained from protein hydrolysis are mostly α- amino acid with the α-carbon atom close to the carboxyl group bound with a amino group (-NH2 in), a carboxyl group (-COOH), a hydrogen atom and a side chain group. The side chain can be denoted as letter R, different R leads to different amino acids; the basic structure of the amino acid formula is as follows:
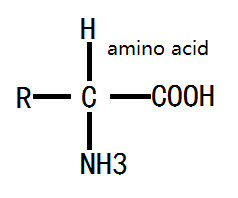
Figure 1: the basic formula of amino acid.
Classification of amino acids according to the polarity of the R, amino acids can be classified into non-polar R group amino acids, uncharged polar R groups of amino acid and positively charged amino acids and negatively charged R-group amino acid. The first category includes alanine, leucine, isoleucine, valine, proline, phenylalanine, and methionine; the second category include glycine, serine, threonine, cysteine and casein acid; the third category include lysine, arginine, and threonine; fourth category include aspartic acid and glutamic acid.
In livestock production, according to different classification methods, amino acids can be divided into essential amino acids and non-essential amino acids, limiting amino acids and non-limiting amino acids.
Isomer: except the glycine (for which, the R group is a hydrogen atom), the alpha-carbons of all the rest amino acids [alpha] are asymmetric carbon atoms, thus having stereoisomers and therefore being optically active. According to the configuration of the α-carbon atom, amino acids can be divided into L-type and D-type. Amino acids originating from the proteolysis of animals and plants are all L-type. Amino acids produced by chemical synthesis and fermentation contain L-amino acids, D-amino acids and DL-amino acids of the mixture of equal amount of L and D type. Animal enzyme system can only directly use the L-amino acid for the protein synthesis of tissues. In addition to methionine, D-amino acids and DL-amino acids have very low utilization efficiency and can even not be exploited by animals.
General properties of amino acids
Physical properties natural amino acid appears as a colorless crystalline material with relatively high melting point, usually being above 200 ℃. It is usually soluble in water, insoluble in non-polar organic solvents. However, tyrosine and cystine are insoluble in water; proline and carboxylic proline can be dissolved in ethanol and ether. All kinds of amino acids are soluble in acid and alkali solution.
The acidity and alkalinity of amino acid amino acids are amphoteric electrolytes, being able to form salts with an acid or a base. The carboxyl group and amino group in the same molecule may also generate internal salts. In solution, amino acid is majorly in ionic form. If we adjust the pH of a certain amino acid solution to become nonconductive that the ions neither move towards cathode nor to anode, the pH of the solution in this case is referred as the isoelectric points of this amino acid, etc.
Chemical reactions related to the determination of amino acids
(1) The reaction with nitrous acid; amino acids can interact with nitrate to generate hydroxy acid and water, and quantitative release nitrogen.
(2) The reaction with 2, 4-nitro fluoro-benzene; upon near neutral or slightly alkaline conditions at room temperature, an amino group inside an amino acids molecule can react with 2, 4-nitro fluoro-benzene to generate the 2,4-nitro fluoro-benzene derivatives of amino acids, i.e., yellow DNP- amino acids.
(3) Reaction with dimethylamino-naphthalene sulfonyl chloride (DNS-CL); the fluorescent reagent DNS-CL can bind with the amino group of amino acids to form fluorescent DNS amino acid into the DNS. Lysine, histidine, aspartic acid, tyrosine and other amino acids can form pair DNS- amino acid derivatives of stable properties.
(4) The reaction with ninhydrin; α-amino acid can be oxidized and decomposed by ninhydrin to aldehyde, ammonia and CO2 while ninhydrin will be restored. The released ammonia can have condensation reaction with another molecule of ninhydrin and one molecule of ninhydrin reduction product to become blue-violet compound.
Separation and Determination of amino acids; amino acids are mostly bound amino acids; the first step of separating amino acids is using acid or base to hydrolyze protein, preparing the mixture of amino acids, then further perform partition chromatography and ion exchange chromatography for further separation and determination of amino acids.
- Structure:
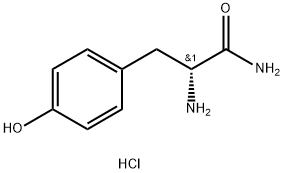
- Chemical Name:(R)-2-AMino-3-(4-hydroxyphenyl)propanaMide hydrochloride
- CAS:117888-79-6
- MF:C9H13ClN2O2
- Structure:
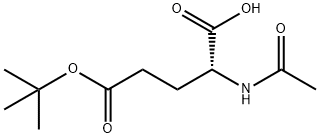
- Chemical Name:Ac-D-Glu(OtBu)-OH
- CAS:1233495-04-9
- MF:C11H19NO5
- Chemical Name:H-Glu-Obzl.HCl
- CAS:
- MF:
- Structure:
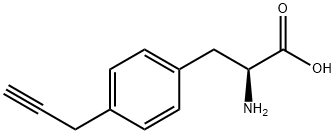
- Chemical Name:H-Tyr(Propargyl)-OH
- CAS:1080496-42-9
- MF:C12H13NO2
- Structure:
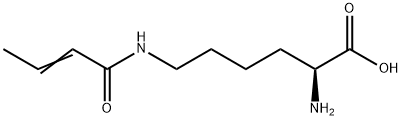
- Chemical Name:Lysine(crotonyl)-OH
- CAS:1338823-35-0
- MF:C10H18N2O3
- Structure:
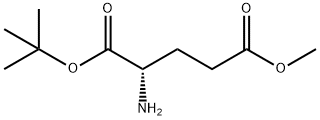
- Chemical Name:H-Glu(OMe)-OtBu
- CAS:79640-72-5
- MF:C10H19NO4
- Structure:
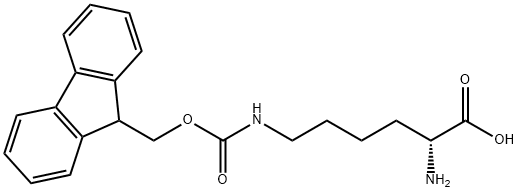
- Chemical Name:N6-FMoc-D-lysine
- CAS:212140-39-1
- MF:C21H24N2O4