Ubiquitin
Ubiquitination refers to the process that ubiquitin molecule (a low molecular weight protein), under the action of a series of special enzymes, perform classification of intracellular proteins from which to choose the target protein molecule, and further conduct specific modification on the target-specific protein. These special enzymes include ubiquitin activating enzyme, bound enzyme, ligase enzymes and degradation enzyme. Ubiquitination plays a very important role in the localization, metabolism, function, regulation and degradation of protein. At the same time, it can also participate into the regulation of almost all life processes including the cell cycle, proliferation, apoptosis, differentiation, metastasis, gene expression, and transcriptional regulation, signal transduction, repairing of damage and inflammation immunity. Ubiquitination has a close relation with the incidence of cancer, cardiovascular and other diseases. Therefore, as a major achievement in the field of biochemical research in recent years, ubiquitination has become the new target for the research and development of new drugs.
The ubiquitination modification (ubiquitylation) process of protein is playing a central role in the regulation of human immune system. Same as phosphorylation process, the process of ubiquitylation is a reversible covalent modification process and is capable of adjusting the stability, functional active status and intracellular localization of the modified protein. Therefore, the ubiquitylation process has also plays a significant role in the development of the human immune system as well as various stages of the immune response such as the initiation, development and the end of the immune response. Recent research results have shown that there are several ubiquitin ligases that have been found to participate into the process of preventing the immune system from attacking the self tissue. The dysfunction of these kinds of ubiquitin ligases is associated with autoimmune diseases.
em should be capable of effective eliminate or limit various kinds of invading pathogens while without attaching the tissue itself. To achieve this goal, it is very necessary for the immune system to conduct fine regulation. As a very important regulatory approach inside the organisms – the ubiquitin modification pathway is of no doubt that plays a highly important role during the regulation process of the immune system. Early research on this topic is mainly focused on NF-κB pathway. In recent years, studies have found that ubiquitylation pathway is capable of activating the NF-κB pathway through several novel signaling pathways, playing a central role in the regulation of NF-κB activation. NF-κB pathway plays a key role in innate immunity and acquired immunity; therefore we have also begun to realize the importance of the regulation of the ubiquitin pathway on the immune system.
Ubiquitin protein is a highly conserved polypeptide consisting of 76 amino acids residues. It can undergo covalent connection with one or more lysine residues contained in the intracellular target proteins under a series enzymatic catalysis of E1, E2, E3 enzymes. Ubiquitin itself also contains seven lysine residues which can connected to each other through these sites, form a multi-ubiquitin chains (poly-ubiquitin chain). The present study has shown that if the poly-ubiquitin chain is connected with the 48th lysine residue of the modified protein, this process will mediate the incorporation of the target protein into the proteasome and further subjecting to degradation. If the poly-ubiquitin chain is connected with other site of the modified protein such as being connected with the first 63 lysine residue, the target protein can exert its function of signaling pathway without being degraded. In addition, some proteins such as histone H2A and H2B, after mono-ubiquitin modification, can also play a role in the regulation and will not be degraded.
Being the same as phosphorylation pathway, ubiquitylation pathway is reversible, which means that the organism can adopt de-ubiquitination enzymes (DUB) to remove the ubiquitin protein modification. This kind of reversible modification pathways is very suitable to be as the regulation mechanism of the immune system. After the target protein has been subject to modification from the ubiquitin pathway, the ubiquitin monomers or polymers connected to the target protein can be recognized and bound by various kinds of ubiquitin domain (UBD). The human proteome contains two kinds of enzymes E1, 50 kinds of E2 enzyme, 600 kinds of E3 enzymes, 90 kinds of DUB enzymes and 20 kinds of UBD, indicating that ubiquitin modification process plays a highly important role in cell regulation. E3 ubiquitin is the key enzyme which determines the substrate specificity during the ubiquitylation process. It can be divided into two categories, namely the E3 enzymes containing the HECT domain and other RING-containing domain or RING-like domain-containing (e.g. U-box or PHD domain) E3 enzymes. Both these two kinds of E3 enzymes have played a key role in immune regulation process.
- Structure:
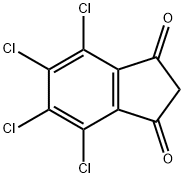
- Chemical Name:TCID
- CAS:30675-13-9
- MF:C9H2Cl4O2
- Structure:
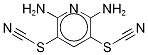
- Chemical Name:PR-619
- CAS:2645-32-1
- MF:C7H5N5S2
- Structure:
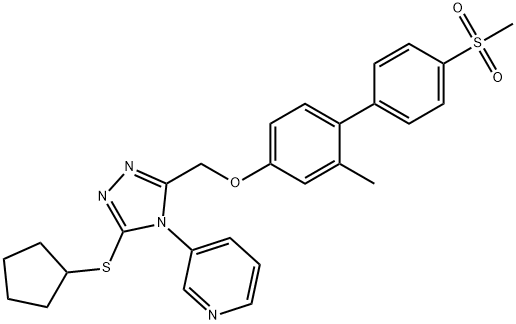
- Chemical Name:NMS 873
- CAS:1418013-75-8
- MF:C27H28N4O3S2
- Structure:
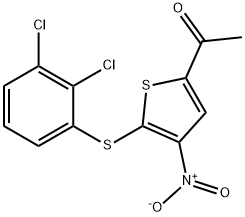
- Chemical Name:P-5091
- CAS:882257-11-6
- MF:C12H7Cl2NO3S2
- Structure:
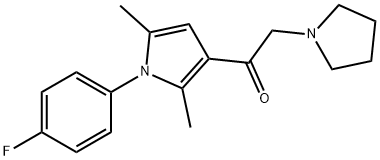
- Chemical Name:1-[1-(4-Fluorophenyl)-2,5-dimethyl-1H-pyrrol-3-yl]-2-(1-pyrrolidinyl)ethanone
- CAS:314245-33-5
- MF:C18H21FN2O
- Structure:
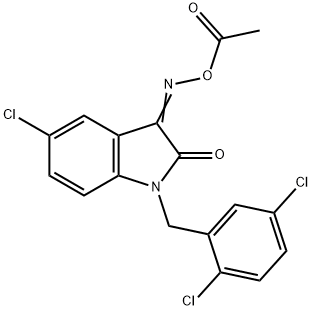
- Chemical Name:LDN-57444
- CAS:668467-91-2
- MF:C17H11Cl3N2O3
- Structure:
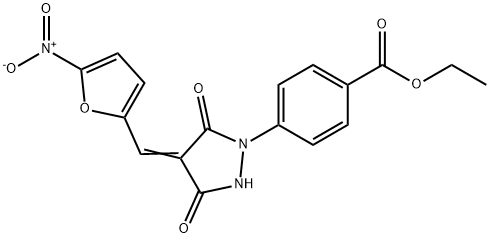
- Chemical Name:PYR 41
- CAS:418805-02-4
- MF:C17H13N3O7
- Structure:
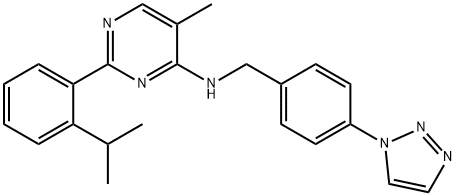
- Chemical Name:ML-323
- CAS:1572414-83-5
- MF:C23H24N6
- Structure:
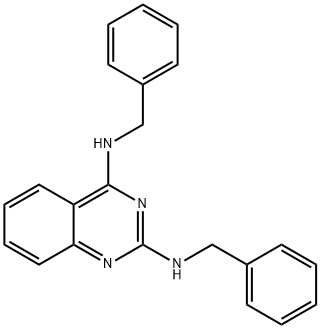
- Chemical Name:DBeQ
- CAS:177355-84-9
- MF:C22H20N4
- Structure:
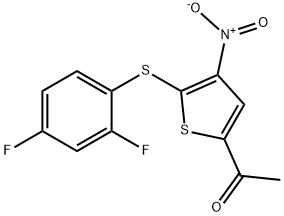
- Chemical Name:P 22077
- CAS:1247819-59-5
- MF:C12H7F2NO3S2
- Structure:
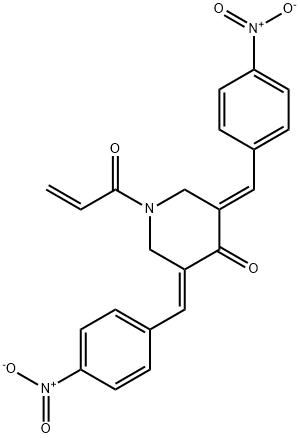
- Chemical Name:b-AP15
- CAS:1009817-63-3
- MF:C22H17N3O6
- Structure:
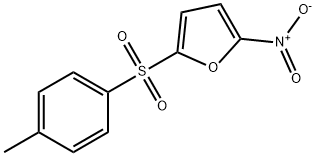
- Chemical Name:NSC697923
- CAS:343351-67-7
- MF:C11H9NO5S