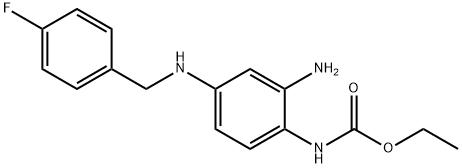
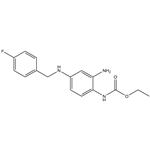
Retigabine was approved in March 2011 by the European Commission for the adjunctive treatment of partial-onset seizures in adults who have epilepsy; in June 2011, the U.S. FDA approved the same drug, known in the United States as ezogabine. Retigabine differs from all currently approved antiepileptic drugs in that it acts as a selective positive allosteric modulator (opener) of KCNQ2-5 potassium channels, which are key regulators of neuronal excitability. The discovery of retigabine was based on modification of an analgesic agent flupirtine that had serendipitously shown potent anticonvulsant activity in animal models of epilepsy. Changing a central 2,3,6-triaminopyridine to a 1,2,4-triaminobenzene decreased analgesic activity while enhancing antiepileptic activity. Retigabine (D-23129) was shown to be a broad spectrum anticonvulsant with oral activity in a variety of animal models. The mechanism of action of retigabine was discovered well after its in vivo activity was recognized. Retigabine is regulated as a Schedule V compound in the United States.