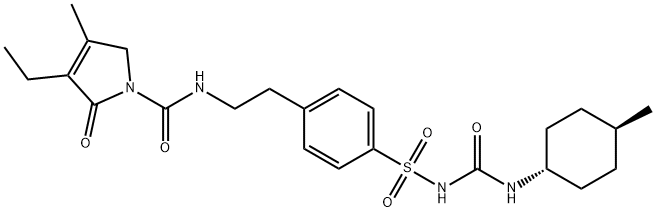
Glimepiride (original trade name Amaryl) is an orally available medium-to-long-acting sulfonylurea antidiabetic drug. It is sometimes classified as either the first third-generation sulfonylurea, or as second-generation. Like all sulfonylureas, glimepiride acts as an insulin secretagogue. It lowers blood sugar by stimulating the release of insulin from functioning pancreatic beta cells and by increasing sensitivity of peripheral tissues to insulin. Glimepiride likely binds to ATP-sensitive potassium channel receptors on the pancreatic cell surface, reducing potassium conductance and causing depolarization of the membrane. Membrane depolarization stimulates calcium ion influx through voltage-sensitive calcium channels. This increase in intracellular calcium ion concentration induces the secretion of insulin. Glimepiride is mainly used to treat patients with type 2 diabetes and can also decrease the chances that someone will develop complications of type 2 diabetes, such as kidney damage, blindness, nerve problems, loss of limbs, sexual function problems and heart attack or stroke. The drug was approved by the FDA in 1995 and is manufactured by Sanofi-Aventis. It can be used along with proper diet and exercise program and may also be used alone or with other antidiabetic medicines if need. The drug is available only with your doctor's prescription.