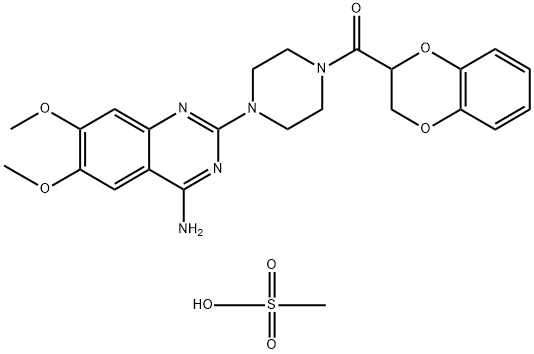
Doxazosin mesylate is a quinazoline compound that is a selective inhibitor of the alpha1 subtype of alpha adrenergic receptors. The chemical name of doxazosin mesylate is 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl) piperazine methanesulfonate.The empirical formula for doxazosin mesylate is C23H25N505 • CH4O3S and the molecular weight is 547.6.
Doxazosin mesylate is freely soluble in dimethylsulfoxide, soluble in dimethylformamide, slightly soluble in methanol, ethanol, and water (0.8% at 25°C), and very slightly soluble in acetone and methylene chloride. Each doxazosin mesylate tablet, for oral administration, contains 1 mg, 2 mg, 4 mg and 8 mg of doxazosin as the free base.
Doxazosin mesylate is a new generation of quinazolone α1 receptor blocker developed by the Pfizer company (United States), It has a long half-life, exerting its effects of dilating the blood vessels, reducing the vascular resistance and lowering the blood pressure through blocking the a 1 receptor. It selectively blocks the a1 adrenergic receptor of the prostate smooth muscle matrix, capsule and bladder neck, alleviating the symptoms of patients of benign prostatic hyperplasia, while having good curing effect on the dysuria caused by simple prostatic hyperplasia. In 1988, doxazosin, as a drug for the treatment of hypertension, had been listed in Denmark. In 1995, the US FDA had approved it for the treatment of benign prostatic hyperplasia. In 2002 September, Doxazosin controlled release tablets had been listed in China, providing a new option for the domestic treatment of benign prostatic hyperplasia. It has been recommended abroad as first-line clinical drugs of anti-hypertension and treatment of prostate disease.