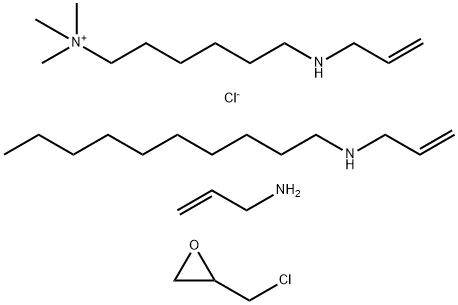
Colesevelam hydrochloride was launched as Welchol (formerly known as Cholestagel)
in the US for the reduction of elevated levels of serum LDL cholesterol and accordingly,
the decrease of the risk of vascular disease in patients with primary hypercholesterolemia.
This orally administered cationic hydrogel is a non-absorbable, water-insoluble polymer of
an hexanaminium chloride with N-(2-propenyl) decanamine, 2-propen-1-amine
hydrochloride and chloromethyloxirane. It acts as a powerful bile acid sequestering agent,
this binding and blockage of bile acids having the end result of compelling the removal of
LDL cholesterol from the blood stream into the liver. In animals fed with a cholesterol-rich
diet for several weeks, colesevelam demonstrated a good maintenance of low serum
cholesterol levels, this activity being significantly greater when compared with
cholestyramine. In several placebo-controlled studies, this agent decreased total
cholesterol levels by 6 to 10% and LDL cholesterol levels by 9 to 20%. Combination
therapy with the co-administration of a HMG-CoA reductase inhibitor (or statin) and
colesevelam produced an additional reduction of 8-16% in LDL-cholesterol levels above
that obtained with the statin alone. Due to its unique water-absorbing hydrogel formulation,
this polymer is not absorbed at all from the GI tract, and thus, it is said to have a lower rate
of side-effects (as the constipating effect) than the previously marketed bile acid
sequestrants. Colesevelam hydrochloride may be used as a monotherapy or as a dual
therapy with statins.