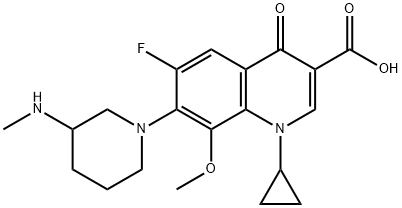
Balofloxacin, a novel orally-active fluoroquinolone antibiotic, was introduced in South
Korea for the treatment of urinary tract infections (UTI). It can be synthetized by reaction of
3-(methylamino)piperidine with the classical 4-quinolone-3-carboxylic acid template. In
vitro antibacterial activity of balofloxacin against gram-positive bacteria (Staphylococcus
aureus including methicillin-resistant S. aureus, Staphylococcus epidermis, Streptococcus
pneumonia, Streptococcus pyrogenes) was almost equal to that of sparfloxacin or
tosufloxacin, in contrast its activity against gram-negative bacteria was 2 times or more
lower. In clinical trials, balofloxacin was well tolerated and showed comparable efficacy to
ofloxacin in patients with UTls. After oral administration, balofloxacin was well absorbed,
and was primarily eliminated unchanged in the urine with an elimination half-life of
approximately 8 h. In animal studies, balofloxacin did not exhibit any phototoxicity.