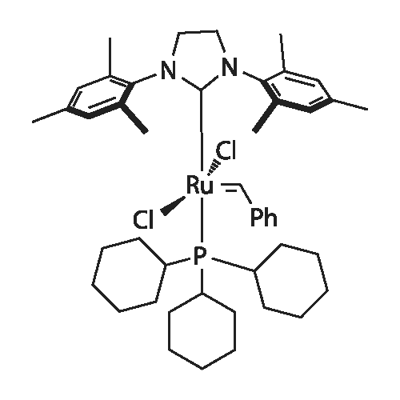
Grubbs catalysts are a series of transition metal carbene complexes used as catalyst for olefin metathesis.1 The Grubbs catalysts are based on a ruthenium atom surrounded by five ligands: two neutral electron-donating entities (e.g., trialkylphosphines, N-heterocyclic carbenes), two monoanionic groups (e.g., halides), and one alkylidene moiety (e.g., unsubstituted and substituted methylidenes). L2X2Ru=CHR complexes (where L is a phosphine ligand) were discovered first and are referred to as the first-generation Grubbs catalyst. (L)(L’)X2Ru=CHR complexes (where L is a phosphine ligand and L’ a saturated N-heterocyclic carbene or NHC ligand) are referred to as the second-generation Grubbs catalysts.
The first-generation Grubbs catalysts show attractive functional-group tolerance and handling properties and have been widely used as highly efficient promoters for ring opening metathesis polymerizations, ring-closing metathesis reactions to make disubstituted olefins, ethenolysis (i.e., cleavage of the carbon–carbon double bond), cross-metathesis of terminal olefins, and the preparation of 1,3-dienes via enyne metathesis. These catalysts and analogues are still widely used in important processes, including the ethenolysis of feedstocks derived from bio-renewable seed oils and the manufacture of macrocyclic hepatitis C therapeutics. Nevertheless, the first-generation Grubbs catalysts show limitations with electron-poor and electron-rich double bonds as well as with sterically hindered systems. The second-generation Grubbs catalysts with excellent metathesis activity while retaining the handling characteristics and broad functional-group tolerance of the earlier Grubbs catalysts are thereby developed. At the same time, the second-generation catalysts are stable against moisture and air.