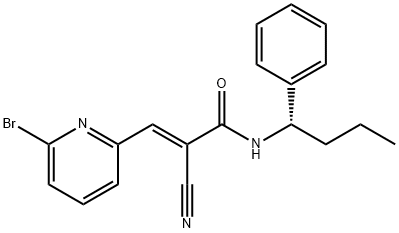
Protein ubiquitination is a dynamic process that can be reversed by deubiquitinating enzymes (DUBs) that remove ubiquitin from proteins, sparing them from degradation by the proteasome. The DUBs have been divided into two subgroups: ubiquitin-specific proteases (USPs) and ubiquitin-specific COOH-terminal hydrolases (UCHs). Recent evidence suggests that several DUBs are activated in tumor cells and many contribute to a transformed phenotype. WP1130 is a second-generation tyrphostin derivative that inhibits the deubiquitinase activity of USP9x, USP5, USP14, and UCH37. At 5 μM, WP1130-mediated inhibition of tumor-activated DUBs induces a rapid accumulation of protein-ubiquitin conjugates, resulting in the formation of aggresomes and apoptosis in a variety of tumor cells. Through this mechanism, WP1130 has been shown to downregulate the antiapoptotic proteins Bcr/Abl and Jak2 and to upregulate the proapoptotic proteins MCL-1 and p53.