| Melting point |
-86 °C |
| Boiling point |
87 °C |
| Density |
1.463 g/mL at 25 °C(lit.) |
| vapor density |
4.5 (vs air) |
| vapor pressure |
61 mm Hg ( 20 °C) |
| refractive index |
n20/D 1.476(lit.) |
| Flash point |
90°C |
| storage temp. |
2-8°C |
| solubility |
Soluble in acetone, ethanol, chloroform, ether (U.S. EPA, 1985), and other organic solvents
including bromoform, carbon tetrachloride, methylene chloride, trichloroethylene, and tetrachloroethylene. |
| form |
Liquid |
| color |
Clear colorless |
| Odor |
Chloroform-like; ethereal. |
| Odor Threshold |
3.9ppm |
| explosive limit |
7.9-99% (v/v) Saturation - at high volume fractions, explosion turns into a decomposition reaction) |
| Water Solubility |
Slightly soluble. 0.11 g/100 mL |
| Merck |
14,9639 |
| BRN |
1736782 |
| Henry's Law Constant |
3.14 at 1.8 °C, 8.47 at 21.6 °C, 19.0 at 40.0 °C, 26.5 at 50 °C, 35.8 at 60 °C, 56.6 at 70 °C
(EPICS-GC, Shimotori and Arnold, 2003) |
| Exposure limits |
TLV-TWA 50 ppm (~270 mg/m3)
(ACGIH), 100 ppm (MSHA and OSHA);
TLV-STEL 200 ppm (ACGIH); ceiling 200
ppm (OSHA); carcinogenicity: Animal Lim ited Evidence, Human Inadequate Evidence
(IARC). |
| Dielectric constant |
3.4(16℃) |
| Stability |
Stable. Incompatible with oxidizing agents, aluminium, magnesium, strong bases, reducing agents. Light-sensitive. Reacts violently with many metals, ozone, potassium nitrate, potassium hydroxide, sodium hydroxide. |
| InChI |
1S/C2HCl3/c3-1-2(4)5/h1H |
| InChIKey |
XSTXAVWGXDQKEL-UHFFFAOYSA-N |
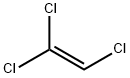
| SMILES |
Cl\C=C(\Cl)Cl |
| LogP |
2.53 at 20℃ |
| CAS DataBase Reference |
79-01-6(CAS DataBase Reference) |
| IARC |
1 (Vol. Sup 7, 63, 106) 2014 |
| NIST Chemistry Reference |
Trichloroethylene(79-01-6) |
| EPA Substance Registry System |
Trichloroethylene (79-01-6) |