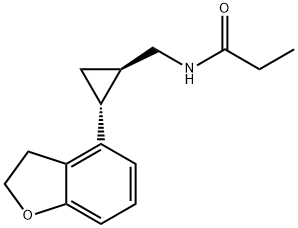
Tasimelteon, which is marketed by Vanda Pharmaceuticals as
Hetlioz� and developed in partnership with Bristol-Myers Squibb,
is a drug that was approved by the US FDA in January 2014 for
the treatment of non-24-hour sleep–wake disorder (also called
Non-24, N24 and N24HSWD). Tasimelteon is a melatonin MT1
and MT2 receptor agonist; because it exhibits a greater affinity to
the MT2 receptor than MT1, is also known as Dual Melatonin
Receptor Agonist.234 Two randomized controlled trials (phases II
and III) demonstrated that tasimelteon improved sleep latency
and maintenance of sleep with a shift in circadian rhythms, and
therefore has the potential to treat patients with transient insomnia
associated with circadian rhythm sleep disorders. Preclinical
studies showed that the drug has similar phase-shifting properties
to melatonin, but with less vasoconstrictive effects.