Telluric acid, H6TeO6, is obtained in the form of its salts
when tellurium is fused with potassium nitrate plus
carbonate, or by the oxidizing action of chlorine on a tellurite
in alkaline solution. The free acid may be obtained
by decomposing the barium salt with sulfuric acid and
concentrating the solution.
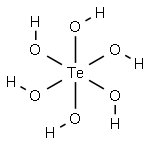
where tellurium is in an octahedral coordination.
Telluric acid, Te(OH)6, has the CAS number of 7803-68-
1 and a molecular weight of 229.6425 g/mol. It is a white,
monoclinic crystal with a density of 3.07 g/cm3. Its
melting point is 136°C and its solubility in water is
50.1 g/100 ml.
It is also formed when tellurium dioxide is oxidized
by hydrogen peroxide in caustic solution (A. Gutbier,
Zest. Anorg. Chem., 1904, 40, p. 260), and perhaps best
of all by oxidizing tellurium with a mixture of nitric
and chromic acids. It crystallizes as prisms, which lose
their water of hydration at 160°C. The tellurates of the
alkaline earth metals are more or less soluble in water,
those of the other metals being very sparingly or almost
insoluble in water. Some tellurates exist in two forms,
a colorless form soluble in water and acids, and a yellow
form insoluble in water and acids.