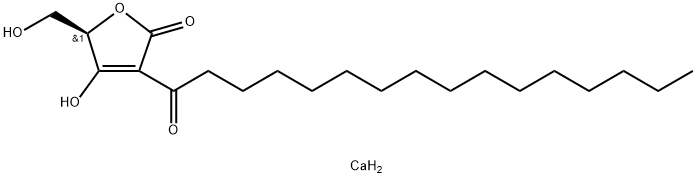
Protein tyrosine phosphatases (PTPs) remove phosphate from tyrosine residues of cellular proteins. Reversible phosphorylation catalyzed by the coordinated actions of protein tyrosine kinases and phosphatases is key to the regulation of the signaling events that control cell growth and proliferation, differentiation, and survival or apoptosis, as well as adhesion and motility. RK-682, a bioactive compound originally isolated from the fermentation of Streptomyces sp. 88-682, is an inhibitor of the PTPs. It inhibits the phosphorylation of CD45 and VHR with IC50 values of 54 and 2 μM, respectively, and arrests cell cycle progress at the G1/S transition. It is also reported to inhibit heparanase (IC50 = 17 μM), an endo-β-D-glucuronidase involved in tumor cell invasion and angiogenesis. RK-682 (calcium salt) is a less soluble version of the free acid.