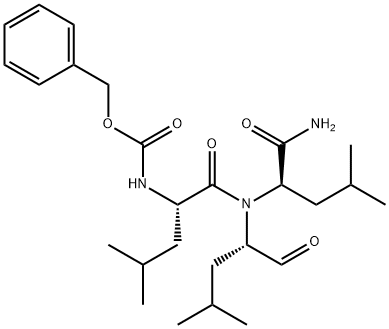
The ubiquitin-proteasome pathway plays an integral role in the selective degradation of intracellular proteins. While important for clearing damaged or mis-folded proteins, this proteolytic pathway also regulates the availability of key proteins involved in the control of inflammatory processes, cell cycle regulation, and gene expression. (R)-MG132 is a potent, reversible, and cell permeable proteasome inhibitor. After treatment for one hour at 100 nM, it inhibits 50% and 31% of proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells, respectively. The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities compared to (S)-MG132 (IC50s = 0.22 versus 0.89 μM (ChTL); 34.4 versus 104.43 μM (TL); 2.95 versus 5.70 μM (PGPH), respectively).