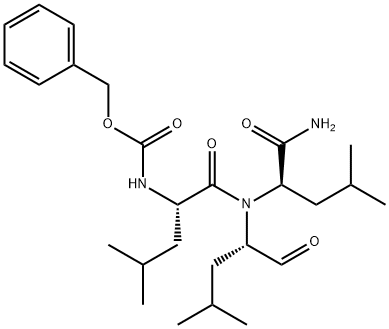
The ubiquitin-
proteasome pathway plays an integral role in the selective degradation of intracellular proteins. While important for clearing damaged or mis-
folded proteins, this proteolytic pathway also regulates the availability of key proteins involved in the control of inflammatory processes, cell cycle regulation, and gene expression.
1,2 (R)-
MG132 is a potent, reversible, and cell permeable proteasome inhibitor. After treatment for one hour at 100 nM, it inhibits 50% and 31% of proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells, respectively.
3 The (R)-
MG132 stereoisomer is a more effective inhibitor of chymotrypsin-
like (ChTL), trypsin-
like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities compared to (S)-
MG132 (IC
50s = 0.22
versus 0.89 μM (ChTL); 34.4
versus 104.43 μM (TL); 2.95
versus 5.70 μM (PGPH), respectively).
3