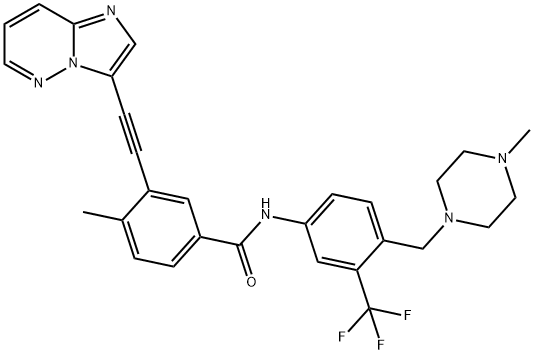
Ponatinib is an orally available, pan-Bcr-Abl tyrosine kinase inhibitor developed for the treatment of an advanced phase of chronic myeloid leukemia in which mutations of the kinase endow resistance to other Bcr-Abl inhibitors. Ponatinib potently inhibits native Bcr-Abl (IC50 = 0.37 nM) as well as various clinically important mutant forms including Bcr-AblT315I (IC50 = 2 nM). It can also inhibit c-Src (IC50 = 5.4 nM) and VEGFR, FGFR, and PDGFR receptor tyrosine kinases (IC50s = 1-2 nM), yet is >1,000-fold selective against family members of Aurora kinase, insulin receptor kinase, or cyclin-dependent kinase. Ponatinib has been shown to inhibit proliferation of Ba/F3 cells expressing native (IC50 = 0.5 nM) or mutant (IC50s = 0.5-36 nM) Bcr-Abl by inducing apoptosis. In a Ba/F3 Bcr- AblT315I xenograft model, tumor growth was inhibited in mice upon oral dosing at 10-30 mg/kg.