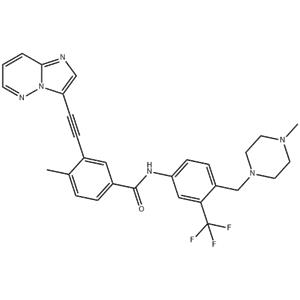
| Description | Ponatinib is a potent, orally available multi-targeted kinase inhibitor with IC50s of 0.37 nM, 1.1 nM, 1.5 nM, 2.2 nM, and 5.4 nM for Abl, PDGFRα, VEGFR2, FGFR1, and Src, respectively. |
|---|
| Related Catalog | Signaling Pathways >> Autophagy >> Autophagy Signaling Pathways >> Protein Tyrosine Kinase/RTK >> Bcr-Abl Signaling Pathways >> Protein Tyrosine Kinase/RTK >> FGFR Signaling Pathways >> Protein Tyrosine Kinase/RTK >> PDGFR Signaling Pathways >> Protein Tyrosine Kinase/RTK >> Src Signaling Pathways >> Protein Tyrosine Kinase/RTK >> VEGFR Research Areas >> Cancer |
|---|
| Target | VEGFR2:1.5 nM (IC50) PDGFRα:1.1 nM (IC50) FGFR1:2.2 nM (IC50) c-Kit:12.5 nM (IC50) |
|---|
| In Vitro | Ponatinib (AP24534) potently inhibits native ABL (IC50: 0.37 nM), ABLT315I (IC50: 2.0 nM), and other clinically important ABL kinase domain mutants (IC50: 0.30-0.44 nM). Ponatinib also inhibits SRC (IC50: 5.4 nM) and members of the VEGFR, FGFR, and PDGFR families of receptor tyrosine kinases. Ponatinib potently inhibits proliferation of Ba/F3 cells expressing native BCR-ABL (IC50: 0.5 nM). All BCR-ABL mutants tested remained sensitive to Ponatinib (IC50: 0.5-36 nM) including BCR-ABLT315I (IC50: 11 nM)[1]. Ponatinib (AP24534) inhibits the in vitro kinase activity of FLT3, KIT, FGFR1, and PDGFRα with IC50 values of 13, 13, 2, and 1 nM, respectively. Ponatinib inhibits phosphorylation of all 4 RTKs in a dose-dependent manner, with IC50 values between 0.3 to 20 nM. Consistent with these activated receptors being important in driving leukemogenesis Ponatinib also potently inhibits the viability of all 4 cell lines with IC50 values of 0.5 to 17 nM. In contrast, the IC50 for inhibition of RS4;11 cells which express native (unmutated) FLT3, is more than 100 nM[2]. |
|---|
| In Vivo | In a survival model in which mice are instead injected with Ba/F3 BCR-ABLT315I cells, administration of Dasatinib at doses as high as 300 mg/kg has no effect on survival time. By contrast, treatment with Ponatinib (AP24534) prolongs survival in a dose-dependent manner. Ponatinib dosed orally for 19 days at 5, 15, and 25 mg/kg prolongs median survival to 19.5, 26, and 30 days, respectively compare to 16 days for vehicle-treated mice (p<0.01 for all three dose levels). The anti-tumor activity of Ponatinib (AP24534) is further assessed in a xenograft model in which Ba/F3 BCR-ABLT315I cells are injected subcutaneously into mice. Tumor growth is inhibited by Ponatinib in a dose-dependent manner compare to vehicle-treated mice, with significant suppression of tumor growth upon daily oral dosing at 10 and 30 mg/kg (%T/C = 68% and 20%, respectively; p<0.01 for both dose levels). Daily oral dosing of 50 mg/kg Ponatinib causes significant tumor regression (%T/C = 0.9%, p<0.01), with a 96% reduction in mean tumor volume at the final measurement compared to the start of treatment. Ponatinib is well tolerated at all efficacious dose levels for the duration of the study; maximal decreases in body weight are <5%, <5%, and <12% for the 10, 30, and 50 mg/kg dose groups, respectively, with no signs of overt toxicity[1]. Ponatinib (1-25 mg/kg) is administered orally, once daily for 28 days, to mice bearing MV4-11 xenografts. Ponatinib potently inhibits tumor growth in a dose-dependent manner. Administration of 1 mg/kg, the lowest dose tested, leads to significant inhibition of tumor growth (TGI=46%, P<0.01) and doses of 2.5 mg/kg or greater results in tumor regression[2]. |
|---|
| Cell Assay | Ba/F3 cell lines are distributed in 96-well plates (4×103 cells/well) and incubated with escalating concentrations of Ponatinib for 72 hr. The inhibitor ranges used are: 0-625 nM for cells expressing BCR-ABL and 0-10,000 nM for BCR-ABL negative cells. Proliferation is measured using a methanethiosulfonate (MTS)-based viability assay. IC50 values are reported as the mean of three independent experiments performed in quadruplicate. For cell proliferation experiments with CML or normal primary cells, mononuclear cells are plated in 96-well plates (5×104 cells/well) over graded concentrations of Ponatinib (0-1000 nM) in RPMI supplemented with 10% FBS, L-glutamine, penicillin/streptomycin, and 100 μM β-mercaptoethanol. Following a 72 hr incubation, cell viability is assessed by subjecting cells to an MTS assay[1]. |
|---|
| Animal Admin | Mice[1] For Ba/F3 survival model, Ba/F3 cells expressing native BCR-ABL or BCR-ABLT315I are injected into the tail vein of female SCID mice (100 μL of a 1×107 cells/mL suspension in serum-free medium). Beginning 72 hr later mice are treated once daily by oral gavage with vehicle (25 mM citrate buffer, pH 2.75), Ponatinib, or Dasatinib for up to 19 consecutive days. Moribund animals are sacrificed as per IACUC guidelines. On necropsy, mice have marked splenomegaly due to tumor cell infiltration. Survival data are analyzed using Kaplan-Meier method, and statistical significance is evaluated with a Log-rank test comparing the survival time of each treatment group with the vehicle group. For Ba/F3 Tumor Model, Ba/F3 BCR-ABLT315I cells are implanted subcutaneously into the right flank of female nude mice (100 μL of a 1×107 cells/mL cell suspension in serum-free medium). Mice are randomized to treatment groups when the average tumor volume reaches approximately 500 mm3. Mice are treated once daily by oral gavage with vehicle (25 mM citrate buffer, pH 2.75) or Ponatinib for up to 19 consecutive days. Tumor volume (mm3) is calculated. To determine tumor growth inhibition when the treatment period is finished, mean tumor volume for treatment group/mean tumor volume for control group (%T/C) is calculated at the final measurement. |
|---|
| References | [1]. O'Hare T, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell, 2009, 16(5), 401-412. [2]. Gozgit JM, et al. Potent activity of ponatinib (AP24534) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies. Mol Cancer Ther, 2011, 10(6), 1028-1035. [3]. Uchida T, et al. Hes1 upregulation contributes to the development of FIP1L1-PDGRA-positive leukemia in blast crisis. Exp Hematol. 2014 May;42(5):369-379.e3. |
|---|


 China
China