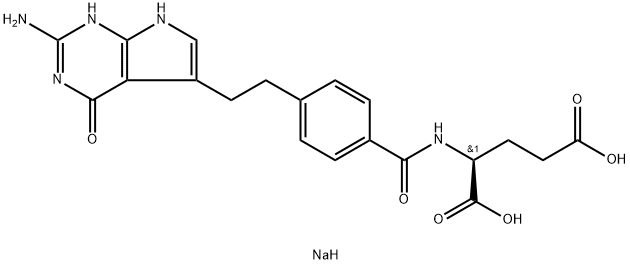
Pemetrexed, a pyrrolo[2,3-d]pyrimidine-based antifolate that disrupts cell replication
by inhibiting multiple folate-dependent metabolic processes, was initially developed
and launched in the US for the treatment of malignant pleural
mesothelioma in conjunction with cisplatin. Patients who are not candidates for
surgery may benefit from this combination therapy. Clinical data demonstrated that
the median overall survival time increased to 12.1 months, compared with 9.3
months for patients receiving cisplatin alone. In August of 2004, the FDA also
approved pemetrexed as a second-line treatment of non-small-cell lung cancer
(NSCLC). While median survival is comparable to the standard second-line treatment
docetaxel, the improved toxicity profile (significant reduction in neutropenia)
accelerated the approval for NSCLC. Its effectiveness as an anticancer drug is
derived from its ability to gain internal cell access via the reduced folate carrier and
membrane folate binding protein transport systems. Once inside, pemetrexed undergoes
polyglutamation, and the resultant polyglutamate forms (predominantly
the pentaglutamate) inhibit the folate-dependent enzymes thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide
formyltransferase (GARFT). Against recombinant human TS, pemetrexed has a
Ki of 109nM while the triglutamate and pentaglutamate forms have Ki values of
1.6nM and 1.3 nM, respectively. All forms of pemetrexed display similar potency
against recombinant human DHFR (7 nM), but the pentaglutamate form is significantly
more potent against recombinant murine GARFT than the parent
(Ki=65nM versus 9.3μM). The selectivity of pemetrexed may be explained by the
fact that polyglutamation is more likely to occur in cancer cells compared to normal
cells while its prolonged duration of action may be attributed to decreased cellular
efflux of the polyglutamate forms. While several different routes have provided
pemetrexed, one of the most efficient exploits the propensity of 2,6-diamino-3Hpyrimidin-
4-one to undergo Michael additions at its unsubstituted C-5 position.
Using ethyl 4-(4-nitrobut-3-enyl)benzoate as the Michael acceptor, the resulting
adduct is then converted to the ultimate precursor for glutamyl coupling via a onepot,
three-step process (Nef reaction to transform the nitro to the aldehyde, intramolecular
condensation to afford the pyrrole, and saponification of the ethyl ester). A
typical treatment regimen involves intravenous administration of pemetrexed, infused
over ten minutes, at a dose of 500mg/m2 followed by a thirty minute wash-out period
and then cisplatin intravenously over two hours at a dose of 75mg/m2. Both drugs are
given on Day 1 of a 21-day cycle. In order to reduce treatment-related hematological
and GI toxicity, patients are instructed to take folic acid and vitamin B12 as a prophylactic
measure. Pretreatment with a corticosteroid is also recommended to prevent
possible skin rashes. Pemetrexed is primarily excreted intact in the urine, with 70–90%
of the dose being recovered within 24 hours of administration. The half-life of pemetrexed
is 3.5 hours in patients with normal renal function, and the total systemic
clearance is 91.8mL/min. As expected, clearance decreases as renal impairment increases.
The drug’s plasma protein binding is 81%, and it has a steady state volume of
distribution of 16.1 L. The pharmacokinetics of pemetrexed is linear with dose and
remains unchanged over multiple treatment cycles. While in vitro studies suggest that
pemetrexed would not interfere with drugs metabolized by CYP3A4, CYP2D6,
CYP2C9, and CYP1A2, ibuprofen (400mg q.d.) does reduce pemetrexed clearance by
20%. Caution should, therefore, be taken when administering pemetrexed concurrently
with ibuprofen to patients with renal insufficiency and should not be given at
all to patients whose creatinine clearance is <45mL/min.
.