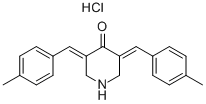
Protein ubiquitination is a dynamic process that can be reversed by deubiquitinating enzymes (DUBs) that remove ubiquitin from proteins, sparing them from degradation by the proteasome. The DUBs have been divided into various groups, the largest being the ubiquitin-specific proteases (USPs). NSC 632839 inhibits USP2 and USP7 as well as the ubiquitin-like SUMO peptidase SENP2 with EC50 values of 45, 37, and 9.8 μM, respectively. It is reported to inhibit apoptosis through a Bcl-2-dependent and apoptosome-independent pathway of caspase activation (IC50 = 15.7 μM in cells sensitized by E1A, an adenoviral oncogene).