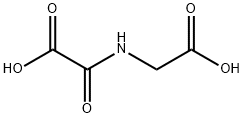
The jumonji domain-containing protein 2 (JMJD2) subfamily of histone demethylases have been shown to catalyze demethylation of the methylated forms of histone 3 lysine 9 (H3K9) and H3K36 in vitro and in cells. Because histone demethylases are implicated in certain diseases, including cancer, selective inhibitors are candidate anticancer agents as well as potential tools for elucidating the biological functions of JMJDs. N-Oxalylglycine, the amide analog of α-ketoglutarate, is a cell permeable inhibitor of α-ketoglutarate-dependent enzymes, including JMJD2A, JMJD2C, and JMJD2E (IC50s = 250, 500, and 24 μM, respectively). It can also inhibit the prolyl hydroxylase domain-containing proteins PHD1 and PHD2 with IC50 values of 2.1 and 5.6 μM, respectively.