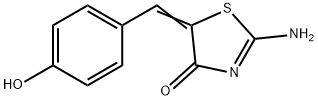
The Mre11-Rad50-Nbs1 (MRN) complex acts as a DNA damage sensor, maintains genome stability during DNA replication, and activates and recruits ataxia-telangiectasia mutated (ATM) to damaged DNA. The genes that encode the MRN proteins are all essential for cell viability, so functional studies of MRN cannot involve deletion of these genes. Mirin is an inhibitor of MRN, inhibiting MRN-dependent phosphorylation of histone H2AX (IC50 = 66 μM). Through its effects on MRN, mirin prevents activation of ATM. Inhibition of MRN centers on the ability of mirin to block the nuclease activity of Mre11, rather than altering DNA-binding or MRN complex formation. In cells, mirin induces G2 arrest, abolishes the radiation-induced G2/M checkpoint, and prevents homology-directed repair of DNA damage.