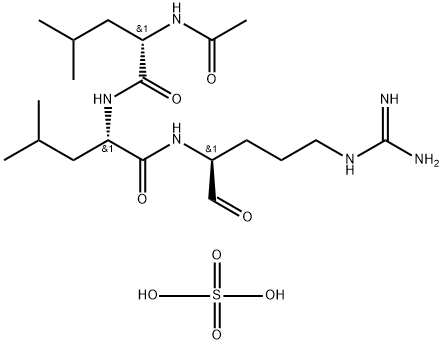
Leupeptin is a reversible inhibitor of cysteine, serine, and threonine proteases that is produced naturally by Streptomyces. It has been reported to inhibit cathepsin B (Ki = 6 nM), calpain (Ki = 10 nM), trypsin (Ki = 35 nM), plasmin (Ki = 3.4 μM), and kallikrein (Ki = 19 μM), and has no effect against chymotrypsin, elastase, renin, or pepsin. Leupeptin has been shown to be protective of auditory hair cells exposed to disruptive noise or ototoxic aminoglycoside-type antibiotics. It is also widely used as a protein purification tool to prevent proteases present in tissue samples from degrading the protein of interest.