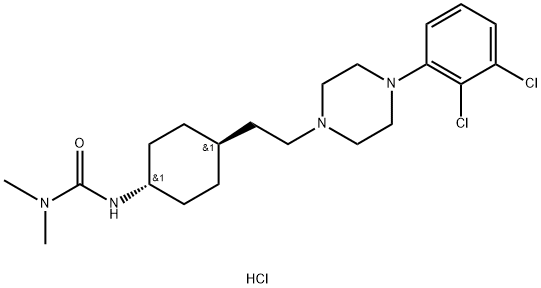
Cariprazine
hydrochloride (IX) is an oral, brain-penetrant, atypical
antipsychotic developed by the Hungarian pharmaceutical
firm Gedeon Richter. It was approved by the FDA in September 2015 for treatment of schizophrenia and for the
acute treatment of manic or mixed episodes of bipolar I
disorder. While the precise mechanism of action of
cariprazine is unknown, its antipsychotic and procognitive
effects may be mediated through partial agonism at dopamine
D2/D3 and serotonin 5-HT1A receptors as well as antagonism
at serotonin 5-HT2A receptors. Unlike many antipsychotics,
cariprazine displays particular selectivity for the D3 receptor
(D3, Ki = 0.085 nM; D2L, Ki = 0.49 nM; D2S, Ki = 0.69 nM).
Cariprazine is extensively metabolized by CYP3A4 and, to a
lesser extent, CYP2D6; desmethyl and didesmethyl cariprazine,
the primary metabolites, are pharmacologically equipotent to
the parent drug. In clinical trials, cariprazine demonstrated
improvement compared to placebo as measured by
Young Mania Rating Scale (YMRS) total scores in patients with
bipolar mania and by Positive and Negative Syndrome Scale
(PANSS) total scores in patients with schizophrenia. Forest
Laboratories (now Allergan) has exclusive rights to cariprazine
in the U.S. and Canada, while Mitsubishi Pharma Corporation
has exclusive rights to the sale of the drug in Japan and Asia.