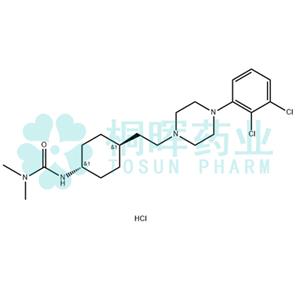
Cariprazine hydrochloride API
Name:Cariprazine hydrochloride
CAS:1083076-69-0
MF:C21H33Cl3N4O
MW:463.87
EINECS:200-077-2
MDL No.:
Properties
storage temp. Store at -20°C
solubility DMSO : 6.67 mg/mL (14.38 mM; Need ultrasonic)
form Powder
color White to off-white
Cariprazine hydrochloride API pharmaceutical export
Cariprazine hydrochloride API pharmaceutical wholesaler
Shortage Cariprazine hydrochloride API Supplies
supply of various Cariprazine hydrochloride API
Cariprazine hydrochloride API Import and Export Wholesaler
wholesale distribution of Cariprazine hydrochloride API
Cariprazine hydrochloride API DISTRIBUTION CHANNELS
Wholesaler Distributor Licensure of Cariprazine hydrochloride API
Certificate of Analysis (COA) for Cariprazine hydrochloride API
Valid Cariprazine hydrochloride API GMP certification
Valid Cariprazine hydrochloride API Manufacturing License
Method of Analysis for Cariprazine hydrochloride API
Material safety data sheet for Cariprazine hydrochloride API
Accelerated stability data for Cariprazine hydrochloride API
Real time stability data for Cariprazine hydrochloride API
Cariprazine hydrochloride API Exporters and Contract manufacturing
Cariprazine hydrochloride in USP, BP, EP with DMF, Tech Pack, GMP, Written Confirmation
Regulatory Documents of Cariprazine hydrochloride
Cariprazine hydrochloride API of manufacturers who are USFDA audited & accredited with GMP, ISO
Cariprazine hydrochloride with DMF/GMP/ISO (all technical documents support)
Cariprazine hydrochloride API in USP/BP/EP pharmacopoeia
Cariprazine hydrochloride Micronised, Injection, & all grades
Cariprazine hydrochloride API factory accredited by USFDA, Health Canada, TGA, UK-MHRA, PIC/S
Cariprazine hydrochloride API with cGMP manufacturing facility、GMP certified facility
Cariprazine hydrochloride API with GLP certified laboratory
Cariprazine hydrochloride API with cGMP & WHO GMP compliant facility
Cariprazine hydrochloride API with R&D and Commercial quantity
Dosage form of Cariprazine hydrochloride API
Cariprazine hydrochloride API U.sPharmacopeia (UsP), in-house specification and/or European Pharmacopoeia (Ph. Eur)
Cariprazine hydrochloride API distributor with a strong supply chain channel
Cariprazine hydrochloride API Provide CEP/COS(certificate of suitability to monograph of European Pharmacopoeia) , EDMF(European Drug Master File),and GMP(Good Manufacturing Practice ),Written Confirmation.
Cariprazine hydrochloride API certificate of analysis
Cariprazine hydrochloride API factory GMP (Goods Manufacturing Practices Certificate )
Cariprazine hydrochloride API Product DML (Drug Manufacturing License)
TOSUN PHARM was established in 1999. In China, it is a collection of drugs, APIs, reference listed drugs, impurities, excipients, intermediates import and export, import registration services, generic drug research and development services, innovative drugs and high-end FDF technology transfer, A group company integrating marketing, academic promotion and cooperative production. With a global procurement, R&D and marketing network, it can quickly meet the needs of product R&D, production and market sales of Chinese and global pharmaceutical companies.
Hot Tags: Cariprazine hydrochloride api, China, suppliers, manufacturers, factory, customized, price, pricelist, in stock

 China
China