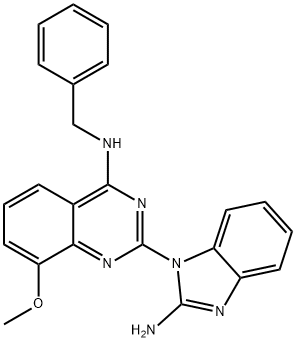
The p97 AAA (ATPase associated with diverse cellular activities) uses energy from ATP hydrolysis to unfold proteins or disassemble protein complexes. p97 is a hexameric protein consisting of two AAA domains (D1 and D2) that form two stacked rings and an N-terminal domain that recruits cofactor/substrate specificity factors. It functions in many regulatory processes to maintain protein homeostasis and has become a therapeutic target for cancer and neurodegenerative diseases. ML-240 is an ATP-competitive inhibitor of the D2 domain of the p97 ATPase (IC50 = 0.11 μM; Ki = 0.22 μM). It disrupts the endoplasmic reticulum-associated degradation and autophagy pathways, preventing the degradation of p97-dependent proteasome substrates (IC50 = 0.9 μM) and causing accumulation of ubiquitin conjugates in nuclear membrane and cytosolic compartments at 5-10 μM. ML-240 has also been shown to block proliferation of HCT15 and SW403 colon cancer cell lines (GI50s = 0.76 and 0.5 μM, respectively) and to rapidly mobilize caspase-3 and -7, inducing apoptosis.