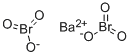
BARIUM BROMATE
$52.65 - $4433.81
- Product nameBARIUM BROMATE
- Cas No13967-90-3
- MFBaBr2O6
- MW393.13
- EINECS237-750-5
- MDL NumberMFCD00014177
| Brand | Packaging | Price | Product name | |
|---|---|---|---|---|
|
American Custom Chemicals Corporation
ING0002413
|
100G | $2471.58 | BARIUM BROMATE |
|
|
American Custom Chemicals Corporation
ING0002413
|
500G | $4433.81 | BARIUM BROMATE |